当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acidity enhancement of α‐carbon of beta diketones via hydroxyl substituents: A density functional theory study
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-12-02 , DOI: 10.1002/poc.4157 Mona Rahimi 1 , Alireza Fattahi 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-12-02 , DOI: 10.1002/poc.4157 Mona Rahimi 1 , Alireza Fattahi 1
Affiliation
|
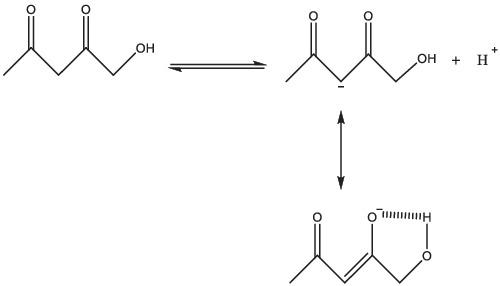
Density functional theory method and B3LYP/6‐311++G(d,p) level of theory were used to determine the acidity of α‐carbon in the hydroxyl derivatives of beta diketones in the gas phase. An investigation of acidity strength in the gas phase indicates that α‐carbon of hydroxyl derivatives of beta diketones become stronger acids than the α‐carbon of beta diketone itself as their conjugate bases gain more stability via both enolate and hydrogen bond formation. Natural bond orbital and quantum theory of atoms in molecules analyses also confirm the role of hydrogen bond interactions on increasing the acidity of α‐carbon of hydroxyl derivatives of beta diketones.
中文翻译:

通过羟基取代基增强β-二酮α-碳的酸度:密度泛函理论研究
密度泛函理论方法和B3LYP / 6-311 ++ G(d,p)水平的理论用于确定气相中β-二酮的羟基衍生物中α-碳的酸度。气相中酸度强度的研究表明,β-二酮的羟基衍生物的α-碳比β-二酮本身的α-碳更强酸,因为它们的共轭碱通过烯醇酸酯和氢键的形成获得更多的稳定性。分子分析中原子的自然键轨道和量子理论还证实了氢键相互作用在增加β-二酮羟基衍生物的α-碳的酸性方面的作用。
更新日期:2021-02-09
中文翻译:

通过羟基取代基增强β-二酮α-碳的酸度:密度泛函理论研究
密度泛函理论方法和B3LYP / 6-311 ++ G(d,p)水平的理论用于确定气相中β-二酮的羟基衍生物中α-碳的酸度。气相中酸度强度的研究表明,β-二酮的羟基衍生物的α-碳比β-二酮本身的α-碳更强酸,因为它们的共轭碱通过烯醇酸酯和氢键的形成获得更多的稳定性。分子分析中原子的自然键轨道和量子理论还证实了氢键相互作用在增加β-二酮羟基衍生物的α-碳的酸性方面的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号