当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation, quality control, and biodistribution assessment of [ 111 In]In‐DOTA‐PR81 in BALB/c mice bearing breast tumors
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2021-01-12 , DOI: 10.1002/jlcr.3897 Sareh Abbas Abadi 1 , Behrouz Alirezapour 2 , István Kertész 3 , Mohammad Javad Rasaee 4 , Javad Mohammadnejad 5 , Malihe Paknejad 6 , Hassan Yousefnia 2 , Samaneh Zolghadri 7
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2021-01-12 , DOI: 10.1002/jlcr.3897 Sareh Abbas Abadi 1 , Behrouz Alirezapour 2 , István Kertész 3 , Mohammad Javad Rasaee 4 , Javad Mohammadnejad 5 , Malihe Paknejad 6 , Hassan Yousefnia 2 , Samaneh Zolghadri 7
Affiliation
|
INTRODUCTION
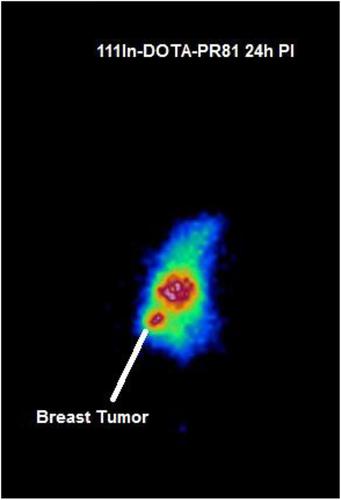
In this study, [111 In]In-DOTA-PR81 was developed and its preliminary preclinical qualifications were assessed for SPECT imaging of breast cancer. METHODS
DOTA-NHS-ester was practiced and successively purified by molecular filtration. The chelate:mAb ratio was determined by spectrophotometry. DOTA-PR81 was radiolabeled with In-111 and its radiochemical yield, in vitro stability, in vitro internalization and immunoreactivity tests were performed. SPECT imaging and tissue counting were applied to evaluate the tissue distribution of [111 In]In-DOTA-hIgG and [111 In]In-DOTA-PR81 in BALB/c mice bearing breast tumors. RESULTS
The radiochemical yield of [111 In]In-DOTA-PR81 complex was >95.0±0.5% (ITLC) and the specific activity was 170±44 MBq/mg. Conjugation reaction resulted in the average number of chelators attached to a mAb (c/a) of 3.4±0.3:1. The radioimmunoconjugate showed immunoreactivity towards MCF7 cell line and MUC1 antigen while its significant in vitro and in vivo stability were investigated over 48 hours respectively (93.0±1.2% in PBS and 84.0±1.3% in human serum). The peak concentration of internalized activity of [111 In]In-DOTA-PR81 was between 4 to 6 h. In comparison to control probes, the complex was accumulated with high specificity and sensitivity at the tumor site. CONCLUSION
Achieved results indicated that [111 In]In-DOTA-PR81 could be contemplated as an appropriate radiotracer for prognostic imaging of antigens in oncology.
中文翻译:

[ 111 In]In-DOTA-PR81 在携带乳腺肿瘤的 BALB/c 小鼠中的制备、质量控制和生物分布评估
引言 在本研究中,开发了 [111 In]In-DOTA-PR81,并评估了其初步临床前资格用于乳腺癌的 SPECT 成像。方法对DOTA-NHS-酯进行实践,并通过分子过滤逐级纯化。通过分光光度法确定螯合物:mAb比率。DOTA-PR81 用 In-111 进行放射性标记,并对其放射化学产量、体外稳定性、体外内化和免疫反应性进行了测试。应用 SPECT 成像和组织计数来评估 [111 In]In-DOTA-hIgG 和 [111 In]In-DOTA-PR81 在携带乳腺肿瘤的 BALB/c 小鼠中的组织分布。结果[111 In]In-DOTA-PR81复合物的放射化学产率>95.0±0.5%(ITLC),比活度为170±44 MBq/mg。缀合反应导致连接到 mAb (c/a) 的平均螯合剂数量为 3.4±0.3:1。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。该复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。该复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。
更新日期:2021-01-12
中文翻译:

[ 111 In]In-DOTA-PR81 在携带乳腺肿瘤的 BALB/c 小鼠中的制备、质量控制和生物分布评估
引言 在本研究中,开发了 [111 In]In-DOTA-PR81,并评估了其初步临床前资格用于乳腺癌的 SPECT 成像。方法对DOTA-NHS-酯进行实践,并通过分子过滤逐级纯化。通过分光光度法确定螯合物:mAb比率。DOTA-PR81 用 In-111 进行放射性标记,并对其放射化学产量、体外稳定性、体外内化和免疫反应性进行了测试。应用 SPECT 成像和组织计数来评估 [111 In]In-DOTA-hIgG 和 [111 In]In-DOTA-PR81 在携带乳腺肿瘤的 BALB/c 小鼠中的组织分布。结果[111 In]In-DOTA-PR81复合物的放射化学产率>95.0±0.5%(ITLC),比活度为170±44 MBq/mg。缀合反应导致连接到 mAb (c/a) 的平均螯合剂数量为 3.4±0.3:1。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。放射免疫偶联物显示出对 MCF7 细胞系和 MUC1 抗原的免疫反应性,同时在 48 小时内分别研究了其显着的体外和体内稳定性(PBS 中为 93.0±1.2%,人血清中为 84.0±1.3%)。[111 In]In-DOTA-PR81 的内化活性峰值浓度在 4 到 6 小时之间。与对照探针相比,复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。该复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。该复合物在肿瘤部位以高特异性和敏感性积累。结论 所取得的结果表明,[111 In]In-DOTA-PR81 可以作为一种合适的放射性示踪剂用于肿瘤学中抗原的预后成像。











































 京公网安备 11010802027423号
京公网安备 11010802027423号