当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Decomposition of 1,3,5,5‐Tetranitrohexahydro‐Pyrimidine: A New Type of Autocatalysis that Persists at High Temperatures
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-12-03 , DOI: 10.1002/prep.202000259 Valery P. Sinditskii 1 , Anastasia D. Smirnova 1 , Tuan Q. Vu 1 , Sergey A. Filatov 1 , Valery V. Serushkin 1 , Gennady F. Rudakov 1
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2020-12-03 , DOI: 10.1002/prep.202000259 Valery P. Sinditskii 1 , Anastasia D. Smirnova 1 , Tuan Q. Vu 1 , Sergey A. Filatov 1 , Valery V. Serushkin 1 , Gennady F. Rudakov 1
Affiliation
|
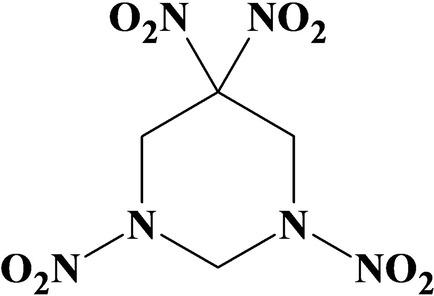
The thermal stability of 1,3,5,5‐ tetranitrohexahydropyrimidine (TNDA) in liquid phase under isothermal conditions was studied. It was established that the TNDA decomposition (kliq=3.1 ⋅ 1021⋅exp(−26865/T), Ea=223.4 kJ mol−1) is accompanied by strong autocatalysis (kcat=9.8 ⋅ 1014⋅exp(‐18056/T), Ea=150.2 kJ mol−1). The mechanism of autocatalysis was proposed. The essence of autocatalysis is the oxidation of TNDA by decomposition products, followed by the destruction of the molecule. An unusual feature of this autocatalysis is that, in contrast to autocatalysis of nitroesters, the process does not disappear at high temperatures, but rather determines the kinetics of heat release in the combustion wave. The surface temperature and combustion mechanism of TNDA were established through thermocouple studies. It was shown that the autocatalysis reaction at the surface temperature controls the burning rate.
中文翻译:

1,3,5,5-四硝基六氢-嘧啶的热分解:一种在高温下持续存在的新型自催化
研究了1,3,5,5-四硝基六氢嘧啶(TNDA)在等温条件下在液相中的热稳定性。它建立了TNDA分解(K LIQ = 3.1⋅10 21 ⋅exp(-26865 / T),E一= 223.4千焦摩尔-1)(k为伴有强自催化猫= 9.8⋅10 14 ⋅exp( - 18056 / T),E a = 150.2 kJ mol -1)。提出了自催化机理。自催化的本质是分解产物将TNDA氧化,然后破坏分子。这种自催化的一个不寻常的特征是,与硝基酯的自催化相比,该过程在高温下不会消失,而是决定了燃烧波中放热的动力学。通过热电偶研究建立了TNDA的表面温度和燃烧机理。结果表明,在表面温度下的自催化反应控制着燃烧速率。
更新日期:2021-01-11
中文翻译:

1,3,5,5-四硝基六氢-嘧啶的热分解:一种在高温下持续存在的新型自催化
研究了1,3,5,5-四硝基六氢嘧啶(TNDA)在等温条件下在液相中的热稳定性。它建立了TNDA分解(K LIQ = 3.1⋅10 21 ⋅exp(-26865 / T),E一= 223.4千焦摩尔-1)(k为伴有强自催化猫= 9.8⋅10 14 ⋅exp( - 18056 / T),E a = 150.2 kJ mol -1)。提出了自催化机理。自催化的本质是分解产物将TNDA氧化,然后破坏分子。这种自催化的一个不寻常的特征是,与硝基酯的自催化相比,该过程在高温下不会消失,而是决定了燃烧波中放热的动力学。通过热电偶研究建立了TNDA的表面温度和燃烧机理。结果表明,在表面温度下的自催化反应控制着燃烧速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号