当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Progress in the Free and Controlled Radical Homo‐ and Co‐Polymerization of Itaconic Acid Derivatives: Toward Functional Polymers with Controlled Molar Mass Distribution and Architecture
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2020-12-03 , DOI: 10.1002/marc.202000546 Lea Sollka 1, 2 , Karen Lienkamp 1, 2, 3
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2020-12-03 , DOI: 10.1002/marc.202000546 Lea Sollka 1, 2 , Karen Lienkamp 1, 2, 3
Affiliation
|
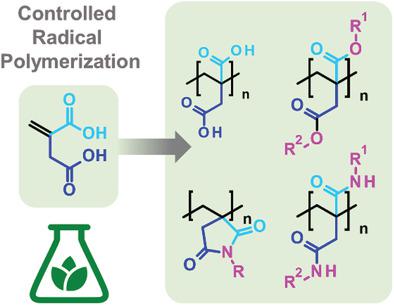
Polymeric derivatives of itaconic acid are becoming increasingly more interesting for research and industry because itaconic acid is accessible from renewable resources. In spite of the structural similarity of poly(itaconic acid derivatives) to poly(methacrylates), they are much less reactive, homopolymerize only sluggishly by free radical polymerization (FRP), and are often obtained with low molar masses and conversions. This has so far limited their use. The reasons for the low reactivity of itaconic acid derivatives (including itaconimides, diitaconates, and diitaconamides) are combined steric and electronic effects, as demonstrated by the body of literature on the FRP homopolymerization kinetics of these monomers which is summarized herein. These problems can be solved to a large extent by using controlled radical polymerization (CRP) techniques, notably atom transfer radical polymerization (ATRP) and reversible addition and fragmentation chain transfer radical polymerization (RAFT). By optimizing the reaction conditions for the ATRP and RAFT of itaconic acid derivatives, in particular the reaction temperature, linear relations between molar mass and conversion are obtained in many cases, and homopolymers with high molar masses and reasonably narrow polydispersity indices become accessible. This review presents the state‐of‐the‐art FRP and CRP of itaconic acid derivatives, and highlights functional polymers obtained by these methods.
中文翻译:

衣康酸衍生物的自由基和自由基自由基共聚和共聚的进展:迈向具有受控摩尔质量分布和结构的功能聚合物
由于衣康酸可从可再生资源获得,因此衣康酸的聚合物衍生物在研究和工业中变得越来越有趣。尽管聚(衣康酸衍生物)与聚(甲基丙烯酸酯)在结构上相似,但是它们的反应性低得多,仅通过自由基聚合(FRP)缓慢地均聚,并且通常以低摩尔质量和转化率获得。迄今为止,这限制了它们的使用。衣康酸衍生物(包括衣康酰亚胺,二衣康酸酯和二衣康酰胺)的低反应性的原因是结合了空间和电子效应,如本文总结的关于这些单体的FRP均聚动力学的文献所证明的。通过使用受控自由基聚合(CRP)技术,尤其是原子转移自由基聚合(ATRP)和可逆加成和断裂链转移自由基聚合(RAFT)技术,可以在很大程度上解决这些问题。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。
更新日期:2020-12-03
中文翻译:

衣康酸衍生物的自由基和自由基自由基共聚和共聚的进展:迈向具有受控摩尔质量分布和结构的功能聚合物
由于衣康酸可从可再生资源获得,因此衣康酸的聚合物衍生物在研究和工业中变得越来越有趣。尽管聚(衣康酸衍生物)与聚(甲基丙烯酸酯)在结构上相似,但是它们的反应性低得多,仅通过自由基聚合(FRP)缓慢地均聚,并且通常以低摩尔质量和转化率获得。迄今为止,这限制了它们的使用。衣康酸衍生物(包括衣康酰亚胺,二衣康酸酯和二衣康酰胺)的低反应性的原因是结合了空间和电子效应,如本文总结的关于这些单体的FRP均聚动力学的文献所证明的。通过使用受控自由基聚合(CRP)技术,尤其是原子转移自由基聚合(ATRP)和可逆加成和断裂链转移自由基聚合(RAFT)技术,可以在很大程度上解决这些问题。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。通过优化衣康酸衍生物的ATRP和RAFT的反应条件,特别是反应温度,在许多情况下获得摩尔质量与转化率之间的线性关系,并且可获得具有高摩尔质量和相当窄的多分散指数的均聚物。这篇综述介绍了衣康酸衍生物的最新FRP和CRP,并重点介绍了通过这些方法获得的功能聚合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号