Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.scitotenv.2020.143910 Zhen Zhang , Tao Wang , Huixue Zhang , Yonghong Liu , Baoshan Xing
|
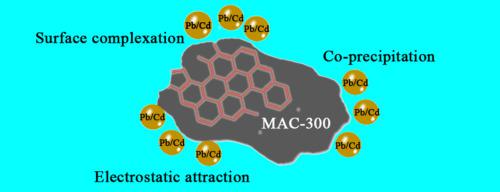
Magnetized activated carbons (MAC) were prepared by activating rape straw powder, and pyrolyzing at different temperatures, then magnetizing activated carbon by hydrothermal method. MAC-300 had the largest adsorption capacity of Pb(II) (253.2 mg/g) and Cd(II) (73.3 mg/g). The adsorption isotherms and kinetics could conform to the Freundlich model and pseudo-second-order kinetic model, respectively, indicating that the adsorptive behavior of the adsorbent mainly depends on the non-uniform active points on the surface of the material. Meanwhile, the thermodynamic parameters showed that the adsorption of Pb(II) and Cd(II) by MAC-300 was a spontaneous and endothermic reaction. The adsorption capacity of MAC-300 could be improved by properly increasing the pH of the original solution. There was competitive adsorption when high-valent ions were present in solution. In combination with various characterizations and comparison tests of samples after adsorption, the adsorption mechanisms include surface electrostatic attraction, surface complexation, and co-precipitation. The results indicated that the MAC material was a potential material to remove heavy metal ions from the aqueous solution.
中文翻译:

磁性活性炭对Pb(II)和Cd(II)的吸附及其机理
通过活化油菜秸秆粉末并在不同温度下热解,然后通过水热法将活性炭磁化来制备磁化活性炭(MAC)。MAC-300具有最大的Pb(II)(253.2 mg / g)和Cd(II)(73.3 mg / g)吸附容量。吸附等温线和动力学可以分别符合Freundlich模型和伪二级动力学模型,这表明吸附剂的吸附行为主要取决于材料表面的不均匀活性点。同时,热力学参数表明,MAC-300对Pb(II)和Cd(II)的吸附是自发吸热反应。通过适当增加原始溶液的pH值可以提高MAC-300的吸附能力。当溶液中存在高价离子时,存在竞争性吸附。结合各种特性和吸附后样品的对比测试,吸附机理包括表面静电吸引,表面络合和共沉淀。结果表明,MAC材料是从水溶液中去除重金属离子的潜在材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号