Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.molmet.2020.101135 Cheng Li 1 , Jing-Jing Xu 1 , Hong-Tao Hu 1 , Chao-Yi Shi 1 , Chuan-Jin Yu 1 , Jian-Zhong Sheng 2 , Yan-Ting Wu 1 , He-Feng Huang 1
|
Objective
Amylin was found to regulate glucose and lipid metabolism by acting on the arcuate nucleus of the hypothalamus (ARC). Maternal high-fat diet (HFD) induces sex-specific metabolic diseases mediated by the ARC in offspring. This study was performed to explore 1) the effect of maternal HFD-induced alterations in amylin on the differentiation of hypothalamic neurons and metabolic disorders in male offspring and 2) the specific molecular mechanism underlying the regulation of amylin and its receptor in response to maternal HFD.
Methods
Maternal HFD and gestational hyper-amylin mice models were established to explore the role of hypothalamic amylin and receptor activity-modifying protein 3 (Ramp3) in regulating offspring metabolism. RNA pull-down, mass spectrometry, RNA immunoprecipitation, and RNA decay assays were performed to investigate the mechanism underlying the influence of maternal HFD on Ramp3 deficiency in the fetal hypothalamus.
Results
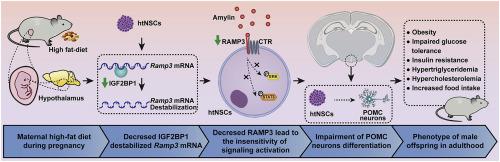
Male offspring with maternal HFD grew heavier and developed metabolic disorders, whereas female offspring with maternal HFD showed a slight increase in body weight and did not develop metabolic disorders compared to those exposed to maternal normal chow diet (NCD). Male offspring exposed to a maternal HFD had hyperamylinemia from birth until adulthood, which was inconsistent with offspring exposed to maternal NCD. Hyperamylinemia in the maternal HFD-exposed male offspring might be attributed to amylin accumulation following Ramp3 deficiency in the fetal hypothalamus. After Ramp3 knockdown in hypothalamic neural stem cells (htNSCs), amylin was found to fail to promote the differentiation of anorexigenic alpha-melanocyte-stimulating hormone-proopiomelanocortin (α-MSH-POMC) neurons but not orexigenic agouti-related protein-neuropeptide Y (AgRP-Npy) neurons. An investigation of the mechanism involved showed that IGF2BP1 could specifically bind to Ramp3 in htNSCs and maintain its mRNA stability. Downregulation of IGF2BP1 in htNSCs in the HFD group could decrease Ramp3 expression and lead to an impairment of α-MSH-POMC neuron differentiation.
Conclusions
These findings suggest that gestational exposure to HFD decreases the expression of IGF2BP1 in the hypothalami of male offspring and destabilizes Ramp3 mRNA, which leads to amylin resistance. The subsequent impairment of POMC neuron differentiation induces sex-specific metabolic disorders in adulthood.
中文翻译:

胰淀素受体不敏感性损害母体高脂饮食喂养小鼠雄性后代的下丘脑 POMC 神经元分化
客观的
发现胰淀素通过作用于下丘脑弓状核 (ARC) 来调节葡萄糖和脂质代谢。母体高脂肪饮食 (HFD) 诱导后代 ARC 介导的性别特异性代谢疾病。本研究旨在探讨 1) 母体 HFD 诱导的胰淀素改变对下丘脑神经元分化和雄性后代代谢紊乱的影响,以及 2) 胰淀素及其受体对母体 HFD 反应调节的特定分子机制.
方法
建立母体HFD和妊娠期高胰淀素小鼠模型,探讨下丘脑胰淀素和受体活性修饰蛋白3(Ramp3)在调节后代代谢中的作用。进行了 RNA 下拉、质谱、RNA 免疫沉淀和 RNA 衰变分析,以研究母体 HFD 对胎儿下丘脑Ramp3缺陷影响的潜在机制。
结果
与母体正常食物 (NCD) 相比,母体 HFD 的雄性后代体重增加并出现代谢紊乱,而母体 HFD 的雌性后代体重略有增加,并且没有出现代谢紊乱。暴露于母体 HFD 的男性后代从出生到成年都有高淀粉血症,这与暴露于母体 NCD 的后代不一致。暴露于母体 HFD 的雄性后代的高淀粉样蛋白血症可能归因于胎儿下丘脑中Ramp3缺陷后的淀粉样蛋白积累。之后RAMP3在下丘脑神经干细胞 (htNSCs) 中敲低,发现胰淀素不能促进厌食性 α-黑色素细胞刺激激素原阿黑皮素原 (α-MSH-POMC) 神经元的分化,但不能促进致厌食性刺鼠相关蛋白-神经肽 Y (AgRP- Npy) 神经元。对相关机制的研究表明,IGF2BP1 可以与 htNSCs 中的 Ramp3 特异性结合并保持其 mRNA 稳定性。HFD 组 htNSCs 中 IGF2BP1 的下调可降低Ramp3表达并导致 α-MSH-POMC 神经元分化受损。
结论
这些发现表明,妊娠期暴露于 HFD 会降低雄性后代下丘脑中IGF2BP1 的表达,并使 Ramp3 mRNA不稳定,从而导致胰淀素抗性。随后 POMC 神经元分化的损伤会在成年期诱导性别特异性代谢紊乱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号