当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
6-Nitro-4,7-dihydroazolo [1,5- a ]pyrimidines: an alternative mechanism of formation and studies of alkylation
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-12-03 , DOI: 10.1007/s10593-020-02839-6 Daniil N. Lyapustin , Evgeny N. Ulomsky , Vladimir L. Rusinov
中文翻译:

6-硝基-4,7-二氢唑啉[1,5-a]嘧啶:烷基化的另一种形成机理和研究
更新日期:2020-12-03
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-12-03 , DOI: 10.1007/s10593-020-02839-6 Daniil N. Lyapustin , Evgeny N. Ulomsky , Vladimir L. Rusinov
|
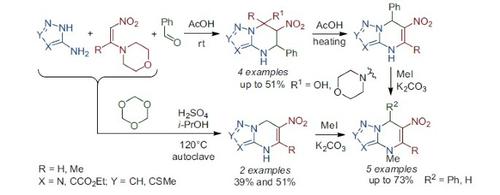
The mechanism of a multicomponent reaction between aminoazoles, 1-morpholino-2-nitroalkenes, and benzaldehyde was studied in acidic medium. Performing the reaction in acetic acid led to the formation of 6-nitro-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrimidines, which subsequently underwent a rearrangement. Reaction conditions were proposed for a multicomponent synthesis with trioxane, and the alkylation reactions of 4,7-dihydroazolo[1,5-a]pyrimidine nitro derivatives were optimized.
中文翻译:

6-硝基-4,7-二氢唑啉[1,5-a]嘧啶:烷基化的另一种形成机理和研究
在酸性介质中研究了氨基唑,1-吗啉代-2-硝基烯烃和苯甲醛之间的多组分反应机理。在乙酸中进行反应导致形成6-硝基-5-苯基-4,5,6,7-四氢吡唑并[1,5- a ]-嘧啶,其随后进行重排。提出了用三恶烷进行多组分合成的反应条件,并优化了4,7-二氢唑并[1,5- a ]嘧啶硝基衍生物的烷基化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号