当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and diastereoselective synthesis of N -substituted 2-acyl-3-aryl-3,5-dihydrofuro[3,2- c ]pyridin-4(2 H )-ones
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-12-03 , DOI: 10.1007/s10593-020-02832-z Evgeny А. Kvetkin , Dmitry V. Osipov , Pavel E. Krasnikov , Vitaly А. Osyanin , Yuri N. Klimochkin
中文翻译:

N-取代的2-酰基-3-芳基-3,5-二氢呋喃[3,2-c]吡啶-4(2 H)-ones的区域和非对映选择性合成
更新日期:2020-12-03
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-12-03 , DOI: 10.1007/s10593-020-02832-z Evgeny А. Kvetkin , Dmitry V. Osipov , Pavel E. Krasnikov , Vitaly А. Osyanin , Yuri N. Klimochkin
|
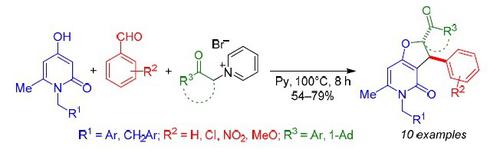
The three-component condensation of pyridinium acylmethylides generated in situ with aromatic aldehydes and 4-hydroxy-6-methylpyridones gave a series of 2-acyl-3-aryl-3,5-dihydrofuro[3,2-c]pyridin-4(2H)-ones. The reaction proceeds diastereoselectively with the formation of trans-isomers and represents a cascade process involving the Knoevenagel condensation, the carbo-Michael reaction, and intramolecular nucleophilic substitution.
中文翻译:

N-取代的2-酰基-3-芳基-3,5-二氢呋喃[3,2-c]吡啶-4(2 H)-ones的区域和非对映选择性合成
与芳族醛和4-羟基-6-甲基吡啶酮原位生成的吡啶鎓甲基吡啶的三组分缩合反应生成一系列2-酰基-3-芳基-3,5-二氢呋喃[3,2 - c ]吡啶-4( 2 H)-一。该反应非对映选择性地进行,形成反式异构体,代表级联过程,涉及Knoevenagel缩合,碳-迈克尔反应和分子内亲核取代。











































 京公网安备 11010802027423号
京公网安备 11010802027423号