当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isothermal Crystallization Monitoring and Time–Temperature-Transformation of Amorphous GDC-0276: Differential Scanning Calorimetric and Rheological Measurements
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.molpharmaceut.0c00776 Sixue Cheng 1 , Paroma Chakravarty 2 , Karthik Nagapudi 2 , Gregory B McKenna 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.molpharmaceut.0c00776 Sixue Cheng 1 , Paroma Chakravarty 2 , Karthik Nagapudi 2 , Gregory B McKenna 1
Affiliation
|
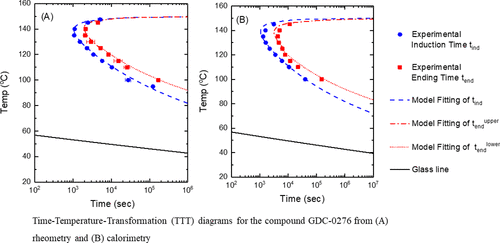
Cold crystallization of amorphous pharmaceuticals is an important aspect in the search to stabilize amorphous or glassy compounds used as amorphous pharmaceutical ingredients (APIs). In the present work, we report results for the isothermal crystallization of the compound GDC-0276 based on differential scanning calorimetric and rheometric measurements. The kinetics of isothermal crystallization from the induction time to the completion of crystallization can be described by the classic Johnson–Mehl–Avrami (JMA) equation. The time–temperature-transformation (TTT) diagrams were constructed for two time points—that of induction and that of completion of crystallization. The results show that the rheological measurement for GDC-0276 has a better overall sensitivity in detection of the early stage nucleation and, consequently, detects the onset of crystallization sooner than does the differential scanning calorimetry. Rheological measurements were also used to obtain the temperature dependence of the viscosity of GDC-0276 and the relevant parameters were used in a modified form of the JMA model to describe the temperature dependence of the crystal induction and completion times, that is, the TTT diagram for the material. In the modification, we assumed that the kinetics followed the viscosity to the 0.75 power as suggested by the recent work of Huang et al. (Huang, C., et al., J. Chem. Phys.2018,149, 054503). The relationship and the possible impact on crystallization kinetics of the break-down of the Stokes–Einstein relation in glass-forming liquids are discussed. From the crystallization kinetics modeling, the solid–liquid interfacial surface tension σSL was obtained for GDC-0276 and was compared with that obtained from the melting point depression measurements of the material confined in nanoporous glasses. The differences between the values from the two methods are discussed.
中文翻译:

无定形 GDC-0276 的等温结晶监测和时间-温度-转化:差示扫描量热和流变测量
无定形药物的冷结晶是稳定用作无定形药物成分 (API) 的无定形或玻璃状化合物的一个重要方面。在目前的工作中,我们报告了基于差示扫描量热和流变测量的化合物 GDC-0276 的等温结晶结果。从诱导时间到结晶完成的等温结晶动力学可以通过经典的 Johnson-Mehl-Avrami (JMA) 方程来描述。时间 - 温度 - 转化(TTT)图是针对两个时间点构建的 - 诱导时间和结晶完成时间。结果表明,GDC-0276 的流变测量在检测早期成核方面具有更好的整体灵敏度,因此,比差示扫描量热法更早地检测到结晶的开始。还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al., 还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al., 还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al.,J.化学。物理。2018, 149, 054503)。讨论了在玻璃形成液体中斯托克斯-爱因斯坦关系分解的关系和对结晶动力学的可能影响。从结晶动力学模型中,获得了 GDC-0276的固-液界面表面张力 σ SL,并将其与从限制在纳米多孔玻璃中的材料的熔点降低测量中获得的结果进行比较。讨论了两种方法的值之间的差异。
更新日期:2021-01-04
中文翻译:

无定形 GDC-0276 的等温结晶监测和时间-温度-转化:差示扫描量热和流变测量
无定形药物的冷结晶是稳定用作无定形药物成分 (API) 的无定形或玻璃状化合物的一个重要方面。在目前的工作中,我们报告了基于差示扫描量热和流变测量的化合物 GDC-0276 的等温结晶结果。从诱导时间到结晶完成的等温结晶动力学可以通过经典的 Johnson-Mehl-Avrami (JMA) 方程来描述。时间 - 温度 - 转化(TTT)图是针对两个时间点构建的 - 诱导时间和结晶完成时间。结果表明,GDC-0276 的流变测量在检测早期成核方面具有更好的整体灵敏度,因此,比差示扫描量热法更早地检测到结晶的开始。还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al., 还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al., 还使用流变测量来获得 GDC-0276 粘度的温度依赖性,并在 JMA 模型的修改形式中使用相关参数来描述晶体诱导和完成时间的温度依赖性,即 TTT 图为材料。在修改中,我们假设动力学遵循 Huang 等人最近的工作建议的 0.75 次幂的粘度。(Huang, C., et al.,J.化学。物理。2018, 149, 054503)。讨论了在玻璃形成液体中斯托克斯-爱因斯坦关系分解的关系和对结晶动力学的可能影响。从结晶动力学模型中,获得了 GDC-0276的固-液界面表面张力 σ SL,并将其与从限制在纳米多孔玻璃中的材料的熔点降低测量中获得的结果进行比较。讨论了两种方法的值之间的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号