当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computation-Assisted Investigation of Polymer Kinetics: Mechanism of the Hybridization of Cobalt-Mediated Radical Polymerization and Atom Transfer Radical Polymerization
Macromolecules ( IF 5.1 ) Pub Date : 2020-12-02 , DOI: 10.1021/acs.macromol.0c02255 Fu-Sheng Wang, Ya-Wen Tsai, Meng-Qin Xie, Chi-How Peng
Macromolecules ( IF 5.1 ) Pub Date : 2020-12-02 , DOI: 10.1021/acs.macromol.0c02255 Fu-Sheng Wang, Ya-Wen Tsai, Meng-Qin Xie, Chi-How Peng
|
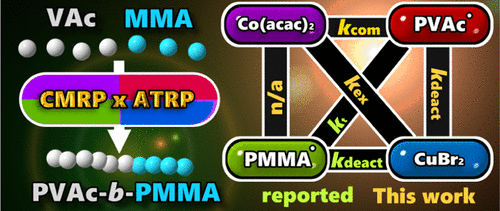
In this study, we combined the kinetic measurement and the computational simulation to build a kinetic model for the hybridization of cobalt-mediated radical polymerization (CMRP) and atom transfer radical polymerization (ATRP), which is a novel method for the one-pot synthesis of block copolymers of less activated monomers and more activated monomers, such as PVAc-b-PMMA and PVAc-b-PSty. The rate constants of the two most important reactions for PVAc radical, the dissociation of PVAc-CoIII(acac)2 and the deactivation with CuII(PMDETA)Br2, have been evaluated at 40 °C as 4.99 × 10–3 s–1 and 4.19 × 106 M–1 s–1, respectively. These two kinetic parameters associated with other rate constants allowed us to build a quantitative model that can simulate the polymerization behavior observed in the hybridization of CMRP and ATRP and thus rationalize the mechanism more precisely.
中文翻译:

聚合物动力学的计算辅助研究:钴介导的自由基聚合和原子转移自由基聚合的杂交机理
在这项研究中,我们将动力学测量和计算仿真相结合,建立了钴介导的自由基聚合(CMRP)和原子转移自由基聚合(ATRP)杂交的动力学模型,这是一种一锅合成的新方法较少活化的单体和较高活化的单体,例如PVAc- b- PMMA和PVAc- b- PSty的嵌段共聚物。对PVAc自由基的两个最重要反应的速率常数,即PVAc-Co III(acac)2的解离和Cu II(PMDETA)Br 2的失活,已在40°C下评估为4.99×10 –3 s –1和4.19×10 6 M–1 s –1。这两个动力学参数与其他速率常数相关联,使我们能够建立一个定量模型,该模型可以模拟在CMRP和ATRP杂交中观察到的聚合行为,从而更精确地合理化机理。
更新日期:2020-12-22
中文翻译:

聚合物动力学的计算辅助研究:钴介导的自由基聚合和原子转移自由基聚合的杂交机理
在这项研究中,我们将动力学测量和计算仿真相结合,建立了钴介导的自由基聚合(CMRP)和原子转移自由基聚合(ATRP)杂交的动力学模型,这是一种一锅合成的新方法较少活化的单体和较高活化的单体,例如PVAc- b- PMMA和PVAc- b- PSty的嵌段共聚物。对PVAc自由基的两个最重要反应的速率常数,即PVAc-Co III(acac)2的解离和Cu II(PMDETA)Br 2的失活,已在40°C下评估为4.99×10 –3 s –1和4.19×10 6 M–1 s –1。这两个动力学参数与其他速率常数相关联,使我们能够建立一个定量模型,该模型可以模拟在CMRP和ATRP杂交中观察到的聚合行为,从而更精确地合理化机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号