当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of the fatty acid coenzyme‐A ligase FadD1 as an interacting partner of FptX in the Pseudomonas aeruginosa pyochelin pathway
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-13 , DOI: 10.1002/1873-3468.14012 Béatrice Roche 1, 2 , Gaëtan L A Mislin 1, 2 , Isabelle J Schalk 1, 2
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-13 , DOI: 10.1002/1873-3468.14012 Béatrice Roche 1, 2 , Gaëtan L A Mislin 1, 2 , Isabelle J Schalk 1, 2
Affiliation
|
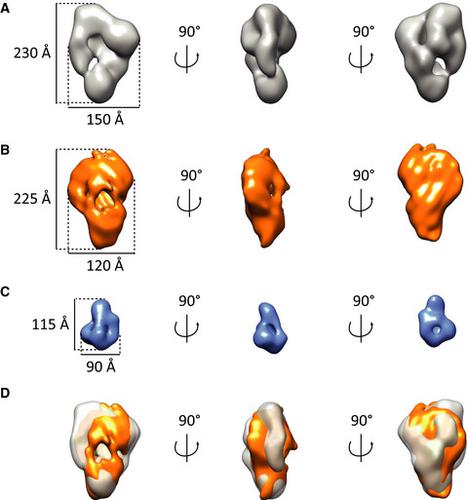
Pseudomonas aeruginosa is one of the most important nosocomial bacteria emerging as a highly multidrug-resistant pathogen. P. aeruginosa produces two siderophores including pyochelin to fulfill its need for iron during infections. We know that both outer and inner membrane proteins FptA and FptX are involved in the ferri-pyochelin uptake, but this process requires increasing molecular and biochemical knowledge. Here, using bacterial two-hybrid and copurification assays we identified the fatty acid coenzyme-A ligase FadD1 as a novel interacting partner of the inner membrane transporter FptX, and found that FadD1 may play a role in pyochelin production. We managed to purify the FadD1-FptX inner membrane complex and obtained low-resolution 3D models, opening the way for future high-resolution structures.
中文翻译:

鉴定脂肪酸辅酶 A 连接酶 FadD1 作为 FptX 在铜绿假单胞菌绿脓杆菌途径中的相互作用伙伴
铜绿假单胞菌是最重要的院内细菌之一,是一种高度多重耐药的病原体。铜绿假单胞菌产生两种铁载体,包括绿脓杆菌素,以满足其在感染期间对铁的需求。我们知道外膜和内膜蛋白 FptA 和 FptX 都参与铁绿脓素吸收,但这个过程需要增加分子和生化知识。在这里,我们使用细菌双杂交和共纯化分析将脂肪酸辅酶 A 连接酶 FadD1 鉴定为内膜转运蛋白 FptX 的新型相互作用伙伴,并发现 FadD1 可能在绿脓素生产中发挥作用。我们设法纯化了 FadD1-FptX 内膜复合物并获得了低分辨率 3D 模型,为未来的高分辨率结构开辟了道路。
更新日期:2020-12-13
中文翻译:

鉴定脂肪酸辅酶 A 连接酶 FadD1 作为 FptX 在铜绿假单胞菌绿脓杆菌途径中的相互作用伙伴
铜绿假单胞菌是最重要的院内细菌之一,是一种高度多重耐药的病原体。铜绿假单胞菌产生两种铁载体,包括绿脓杆菌素,以满足其在感染期间对铁的需求。我们知道外膜和内膜蛋白 FptA 和 FptX 都参与铁绿脓素吸收,但这个过程需要增加分子和生化知识。在这里,我们使用细菌双杂交和共纯化分析将脂肪酸辅酶 A 连接酶 FadD1 鉴定为内膜转运蛋白 FptX 的新型相互作用伙伴,并发现 FadD1 可能在绿脓素生产中发挥作用。我们设法纯化了 FadD1-FptX 内膜复合物并获得了低分辨率 3D 模型,为未来的高分辨率结构开辟了道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号