当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Fused Tricyclic [1,3]oxazino[3,4‐a]indolone and Dihydropyrimido [1,6‐a]indolone via Rh(III)‐catalyzed [3+3] or [4+2] C−H Annulation
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-12-01 , DOI: 10.1002/adsc.202001286 Junyu Chen 1 , Tianshuo Zhong 1 , Xiangyun Zheng 1 , Chuanliu Yin 1 , Lei Zhang 1 , Jian Zhou 1 , Xinpeng Jiang 1 , Chuanming Yu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-12-01 , DOI: 10.1002/adsc.202001286 Junyu Chen 1 , Tianshuo Zhong 1 , Xiangyun Zheng 1 , Chuanliu Yin 1 , Lei Zhang 1 , Jian Zhou 1 , Xinpeng Jiang 1 , Chuanming Yu 1
Affiliation
|
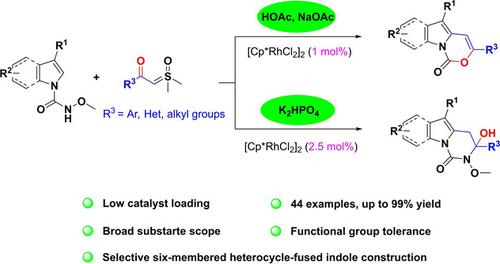
A formation of fused tricyclic [1,3]oxazino[3,4‐a]indol‐1‐ones and dihydropyrimido[1,6‐a]indol‐1(2H)‐ones via Rh(III)‐catalyzed [3+3] or [4+2] annulation of N‐methoxy‐1H‐indole‐1‐carboxamides with sulfoxonium ylides has been developed. These selective annulation reactions were carried out by switching the additives and notable features of this protocol were low catalyst loading and a broad substrate scope providing the corresponding products in up to 99% yields.
中文翻译:

经由Rh(III)催化的[3 + 3]或[4 + 2] CH选择性合成熔融的三环[1,3]恶嗪基[3,4-a]吲哚酮和二氢嘧啶基[1,6-a]吲哚酮环
经由Rh(III)催化的稠合三环[1,3] oxazino [3,4- a ]吲哚-1-酮和二氢嘧啶基[1,6 - a ] indol-1(2 H)-酮的形成[3已开发了N ‐甲氧基-1 H吲哚-1-羧酰胺与亚砜基鎓盐的+3]或[4 + 2]环化反应。这些选择性环化反应是通过切换添加剂来进行的,该方案的显着特征是催化剂用量低,底物范围广,可以以高达99%的产率提供相应的产物。
更新日期:2021-01-19
中文翻译:

经由Rh(III)催化的[3 + 3]或[4 + 2] CH选择性合成熔融的三环[1,3]恶嗪基[3,4-a]吲哚酮和二氢嘧啶基[1,6-a]吲哚酮环
经由Rh(III)催化的稠合三环[1,3] oxazino [3,4- a ]吲哚-1-酮和二氢嘧啶基[1,6 - a ] indol-1(2 H)-酮的形成[3已开发了N ‐甲氧基-1 H吲哚-1-羧酰胺与亚砜基鎓盐的+3]或[4 + 2]环化反应。这些选择性环化反应是通过切换添加剂来进行的,该方案的显着特征是催化剂用量低,底物范围广,可以以高达99%的产率提供相应的产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号