当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium Catalyzed Regioselective β‐C(sp3)−H Functionalization of N‐Alkyl‐N′‐p–nitrophenyl Substituted Piperazines using Aldehydes as Alkylating Agents
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1002/adsc.202001060 V. Murugesh 1, 2 , Apurba Ranjan Sahoo 3 , Mathieu Achard 3 , Gangavaram V. M. Sharma 1 , Christian Bruneau 3 , Surisetti Suresh 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1002/adsc.202001060 V. Murugesh 1, 2 , Apurba Ranjan Sahoo 3 , Mathieu Achard 3 , Gangavaram V. M. Sharma 1 , Christian Bruneau 3 , Surisetti Suresh 1, 2
Affiliation
|
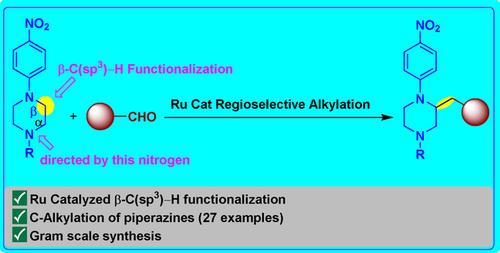
Herein, we disclose a ruthenium‐catalyzed regioselective β‐C(sp3)−H bond functionalization on the piperazine core using aldehydes as alkylating agents. The present transformation appears to go through the dehydrogenation of the piperazine to propagate to enamine in situ, followed by nucleophilic addition to the aldehyde and hydrogenation to result in the regioselective β‐C(sp3)−H alkylation. A variety of aromatic, heteroaromatic, aliphatic aldehydes were employed for the C‐3 alkylation of N‐alkyl‐N′‐p‐nitrophenyl substituted piperazines.
中文翻译:

钌作为醛化剂催化N-烷基-N'-对硝基苯基取代的哌嗪的钌催化区域选择性β-C(sp3)-H官能化
在本文中,我们公开了使用醛类作为烷基化剂在哌嗪核上进行钌催化的区域选择性β-C(sp 3)-H键官能化。目前的转化似乎是通过哌嗪的脱氢反应,使其原位扩散为烯胺,然后向醛中进行亲核加成反应,然后进行氢化反应,从而形成区域选择性的β-C(sp 3)-H烷基化反应。多种芳族,杂芳族,脂族醛的被用于的C-3烷基化ñ -烷基- N' - p硝基苯基取代的哌嗪。
更新日期:2021-01-19
中文翻译:

钌作为醛化剂催化N-烷基-N'-对硝基苯基取代的哌嗪的钌催化区域选择性β-C(sp3)-H官能化
在本文中,我们公开了使用醛类作为烷基化剂在哌嗪核上进行钌催化的区域选择性β-C(sp 3)-H键官能化。目前的转化似乎是通过哌嗪的脱氢反应,使其原位扩散为烯胺,然后向醛中进行亲核加成反应,然后进行氢化反应,从而形成区域选择性的β-C(sp 3)-H烷基化反应。多种芳族,杂芳族,脂族醛的被用于的C-3烷基化ñ -烷基- N' - p硝基苯基取代的哌嗪。










































 京公网安备 11010802027423号
京公网安备 11010802027423号