当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Role of pH in Protein‐Haptenation Reaction: Kinetics and Mechanisms of the Protein‐Haptenation Reactions of Selected Quinones Present in the Environment
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-12-02 , DOI: 10.1002/slct.202003310 Olufunmilayo A. Olusegun 1 , Bice S. Martincigh 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-12-02 , DOI: 10.1002/slct.202003310 Olufunmilayo A. Olusegun 1 , Bice S. Martincigh 1
Affiliation
|
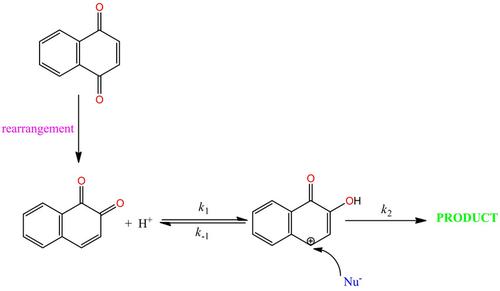
There are several xenobiotics (allergens) in the environment which may induce allergic contact dermatitis (ACD), an occupational and environmental health disease. This health disease is of global concern because it has no cure and can only be managed in affected individuals by preventing them from further exposure to these allergens. The following quinones (Q) are known to be air pollutants: 1,2‐naphthoquinone (1,2‐NQ), 1,4‐naphthoquinone (1,4‐NQ), 9,10‐phenanthraquinone (PQ), and 9,10‐anthraquinone (AQ). There have been studies on the cytotoxicity of these air pollutants, but no kinetic studies have been carried out on them to determine their chemical reactivity and, more especially, their interaction with model proteins so as to ascertain if they can react with skin proteins thereby causing allergic reactions. This article provides details on the kinetics studies carried out on the protein‐haptenation reactions of these quinones with 4‐nitrobenzenethiol (NBT), a model nucleophile, as well as the mechanisms of their reactions. The NBT−Q reactions were investigated under pseudo‐first‐order conditions at various concentration ratios at a temperature of 25 °C at three different pH values by either ultraviolet‐visible (UV‐Vis) or stopped‐flow spectrophotometry, as appropriate. The reaction rate constants obtained for the NBT−Q reactions were in the following order: at pH 7.42: 1,2‐NQ>1,4‐NQ>PQ>AQ while at pH 5.65, the order was observed to be 1,2‐NQ>1,4‐NQ>PQ>AQ and at pH 8.41, it was 1,4‐NQ>PQ>AQ>1,2‐NQ. These trends for the reaction rate constants of the Q at the three pH values can be attributed to inductive effects and the position of oxo‐groups on the phenyl rings. The NBT−Q reactions were further investigated by determining the effect of ionic strength on the reaction rate constants with potassium sulphate as the background electrolyte. Our findings showed that there are no charged species at the transition state of the NBT−Q reactions. The products of the NBT−Q reactions were isolated and characterized by means of UV‐Vis and FTIR spectrophotometry and NMR and TOF‐MS spectrometry. The deduced mechanisms for the NBT−Q reactions were validated by computer simulation with “Simkine3” software.
中文翻译:

理解pH在蛋白质加合反应中的作用:环境中存在的选定醌的蛋白质加合反应的动力学和机理
环境中存在几种异源生物(过敏原),它们可能诱发过敏性接触性皮炎(ACD),这是一种职业和环境健康疾病。这种健康疾病是全球性关注的问题,因为它无法治愈,只能通过防止受影响的人进一步暴露于这些过敏原中来对其进行管理。已知以下醌(Q)是空气污染物:1,2-萘醌(1,2-NQ),1,4-萘醌(1,4-NQ),9,10-菲蒽醌(PQ)和9 ,10-蒽醌(AQ)。已经对这些空气污染物的细胞毒性进行了研究,但是尚未对其进行动力学研究来确定其化学反应性,尤其是它们与模型蛋白的相互作用,以确定它们是否可以与皮肤蛋白反应从而引起过敏反应。本文详细介绍了这些醌与模型亲核试剂4-硝基苯硫醇(NBT)的蛋白质半抗原反应的动力学研究及其反应机理。NBT-Q反应在伪一级条件下在25°C的温度,三个不同的pH值,不同浓度比下通过紫外可见(UV-Vis)或停止流式分光光度法进行了研究。为NBT-Q反应获得的反应速率常数按以下顺序:在pH 7.42:1,2-NQ> 1,4-NQ> PQ> AQ而在pH 5.65时,观察到的顺序是1,2 -NQ> 1,4-NQ> PQ> AQ,在pH 8.41时为1,4-NQ> PQ> AQ> 1,2-NQ。Q在三个pH值下的反应速率常数的这些趋势可归因于感应效应和苯环上氧代基团的位置。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。
更新日期:2020-12-02
中文翻译:

理解pH在蛋白质加合反应中的作用:环境中存在的选定醌的蛋白质加合反应的动力学和机理
环境中存在几种异源生物(过敏原),它们可能诱发过敏性接触性皮炎(ACD),这是一种职业和环境健康疾病。这种健康疾病是全球性关注的问题,因为它无法治愈,只能通过防止受影响的人进一步暴露于这些过敏原中来对其进行管理。已知以下醌(Q)是空气污染物:1,2-萘醌(1,2-NQ),1,4-萘醌(1,4-NQ),9,10-菲蒽醌(PQ)和9 ,10-蒽醌(AQ)。已经对这些空气污染物的细胞毒性进行了研究,但是尚未对其进行动力学研究来确定其化学反应性,尤其是它们与模型蛋白的相互作用,以确定它们是否可以与皮肤蛋白反应从而引起过敏反应。本文详细介绍了这些醌与模型亲核试剂4-硝基苯硫醇(NBT)的蛋白质半抗原反应的动力学研究及其反应机理。NBT-Q反应在伪一级条件下在25°C的温度,三个不同的pH值,不同浓度比下通过紫外可见(UV-Vis)或停止流式分光光度法进行了研究。为NBT-Q反应获得的反应速率常数按以下顺序:在pH 7.42:1,2-NQ> 1,4-NQ> PQ> AQ而在pH 5.65时,观察到的顺序是1,2 -NQ> 1,4-NQ> PQ> AQ,在pH 8.41时为1,4-NQ> PQ> AQ> 1,2-NQ。Q在三个pH值下的反应速率常数的这些趋势可归因于感应效应和苯环上氧代基团的位置。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。通过确定离子强度对硫酸钾作为背景电解质的反应速率常数的影响,进一步研究了NBT-Q反应。我们的发现表明,在NBT-Q反应的过渡态下没有带电物质。通过UV-Vis和FTIR分光光度法以及NMR和TOF-MS光谱对NBT-Q反应产物进行分离和表征。通过使用“ Simkine3”软件进行计算机仿真,验证了推导的NBT-Q反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号