当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating the mechanistic inhibitory discrepancies of novel halogen and alkyl di‐substituted oxadiazole‐based dibenzo‐azepine‐dione derivatives on Poly (ADP‐ribose) Polymerase‐1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-12-22 , DOI: 10.1002/cbdv.202000802 Felix O Okunlola 1 , Opeyemi S Soremekun 1 , Fisayo A Olotu 1 , Mahmoud E S Soliman 1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-12-22 , DOI: 10.1002/cbdv.202000802 Felix O Okunlola 1 , Opeyemi S Soremekun 1 , Fisayo A Olotu 1 , Mahmoud E S Soliman 1
Affiliation
|
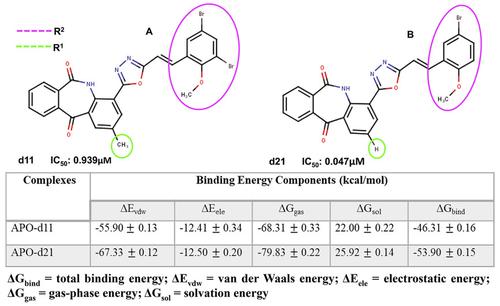
Numerous studies have established the involvement of Poly (ADP‐ribose) Polymerase‐1 (PARP‐1) in cancer presenting it as an important therapeutic target over recent years. Although homology among the PARP protein family makes selective targeting difficult, two compounds [d11 (0.939 μM) and d21 (0.047 μM)] with disparate inhibitory potencies against PARP‐1 were recently identified. In this study, free energy calculations and molecular simulations were used to decipher underlying mechanisms of differential PARP‐1 inhibition exhibited by the two compounds. The thermodynamics calculation revealed that compound d21 had a relatively higher ΔGbind than d11. High involvement of van der Waal and electrostatic effects potentiated the affinity of d21 at PARP‐1 active site. More so, incorporated methyl moiety in d11 accounted for steric hindrance which, in turn, prevented complementary interactions of key site residues such as TYR889, MET890, TYR896, TYR907. Conformational studies also revealed that d21 is more stabilized for interactions in the active site compared to d11. We believe that findings from this study would provide an important avenue for the development of selective PARP‐1 inhibitors.
中文翻译:

研究新型卤素和烷基二取代恶二唑基二苯并氮杂二酮衍生物对聚(ADP-核糖)聚合酶-1 的机械抑制差异
近年来,大量研究证实聚(ADP-核糖)聚合酶-1(PARP-1)参与癌症,将其作为重要的治疗靶点。尽管 PARP 蛋白家族之间的同源性使得选择性靶向变得困难,但最近发现了两种对 PARP-1 具有不同抑制效力的化合物 [d11 (0.939 μM) 和 d21 (0.047 μM)]。在这项研究中,自由能计算和分子模拟被用来破译两种化合物表现出的差异 PARP-1 抑制的潜在机制。热力学计算表明,化合物 d21 的 ΔGbind 比 d11 高。范德华和静电效应的高度参与增强了 d21 在 PARP-1 活性位点的亲和力。更重要的是,在 d11 中掺入的甲基部分导致了空间位阻,反过来,阻止了关键位点残基(如 TYR889、MET890、TYR896、TYR907)的互补相互作用。构象研究还表明,与 d11 相比,d21 在活性位点的相互作用更稳定。我们相信,这项研究的发现将为开发选择性 PARP-1 抑制剂提供重要途径。
更新日期:2020-12-22
中文翻译:

研究新型卤素和烷基二取代恶二唑基二苯并氮杂二酮衍生物对聚(ADP-核糖)聚合酶-1 的机械抑制差异
近年来,大量研究证实聚(ADP-核糖)聚合酶-1(PARP-1)参与癌症,将其作为重要的治疗靶点。尽管 PARP 蛋白家族之间的同源性使得选择性靶向变得困难,但最近发现了两种对 PARP-1 具有不同抑制效力的化合物 [d11 (0.939 μM) 和 d21 (0.047 μM)]。在这项研究中,自由能计算和分子模拟被用来破译两种化合物表现出的差异 PARP-1 抑制的潜在机制。热力学计算表明,化合物 d21 的 ΔGbind 比 d11 高。范德华和静电效应的高度参与增强了 d21 在 PARP-1 活性位点的亲和力。更重要的是,在 d11 中掺入的甲基部分导致了空间位阻,反过来,阻止了关键位点残基(如 TYR889、MET890、TYR896、TYR907)的互补相互作用。构象研究还表明,与 d11 相比,d21 在活性位点的相互作用更稳定。我们相信,这项研究的发现将为开发选择性 PARP-1 抑制剂提供重要途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号