Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-12-02 , DOI: 10.1016/j.cej.2020.127919 Juchao Liu , Wanpeng Chen , Xuebin Hu , Hainan Wang , Yijie Zou , Qiang He , Jun Ma , Caihong Liu , Yao Chen , Xiaoliu Huangfu
|
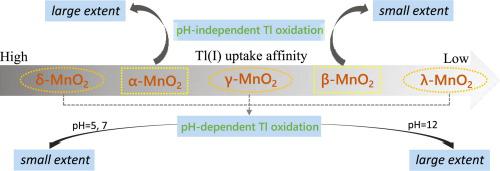
Manganese dioxides (MnO2) with a high affinity for thallium (Tl), strongly influence its fate and toxicity in aquatic environments. The crystal structure of MnO2 plays a critical role in their activity for the uptake of heavy metals. However, few studies have examined the differences in interactions between Tl and various crystal structured MnO2. In this study, the fundamental data on the sorption and oxidative reactivity of Tl(I) by MnO2 with five known crystal structures (δ-, α-, γ-, β-, λ-MnO2) was obtained at different pH values (5, 7, 12). The results showed that the uptake of Tl followed the order of δ-MnO2 > α-MnO2 > γ-MnO2 > β-MnO2 > λ-MnO2, and increased with increasing pH for each MnO2. Moreover, α-MnO2 exhibited the strongest oxidation ability for Tl(I) at all the tested pH values, while β-MnO2 could scarcely oxidize aqueous Tl(I). However, for δ-MnO2, γ-MnO2 and λ-MnO2, the extent of Tl(I) oxidation was much larger at pH 12 than at pH 5 or 7, which was ascribed to the active species of TlOH involved in the redox process. Besides, the sorption of Tl(I) led to a decrease in crystallinity of δ-MnO2 and α-MnO2 but slightly impacted the structure of γ-MnO2, β-MnO2 and λ-MnO2. These findings might contribute to understanding the immobilization processes of Tl by different crystal structured MnO2 at various pH conditions.
中文翻译:

MnO 2晶体结构对hall的吸附和氧化反应的影响(I)
对th(Tl)具有高亲和力的二氧化锰(MnO 2)强烈影响其在水生环境中的命运和毒性。MnO 2的晶体结构在其吸收重金属的活性中起着至关重要的作用。然而,很少有研究检查T1和各种晶体结构的MnO 2之间相互作用的差异。在这项研究中,对铊(I)的吸附和氧化反应用MnO的基本数据2具有五个已知的晶体结构(δ-,α-,γ-,β-,λ-MnO的2在不同pH值获得) (5、7、12)。结果表明,T1的摄取,随后的次序δ-的MnO 2 >α-MnO的2 >γ-MnO的2>β-MnO的2 >λ-MnO的2,以及对于每个增加的MnO pH升高2。此外,α-MnO的2表现出在所有测试的pH值下对铊(I)最强的氧化能力,而β-MnO的2几乎无法氧化铊水溶液(I)。然而,对于δ-的MnO 2, γ-MnO的2和λ-MnO的2,T1的范围(I)的氧化是在pH 12比在pH 5或7,其归因于TlOH的活性种大得多的参与氧化还原过程。此外,T1的吸附(I)导致的结晶度δ-MnO的减少2和α-MnO的2但略有影响γ-MnO的结构2,β-MnO的2和λ-MnO的2。这些发现可能有助于理解在各种pH条件下不同晶体结构的MnO 2对T1的固定过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号