Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-12-02 , DOI: 10.1016/j.cej.2020.127922 Beatriz Sarrión , Antonio Perejón , Pedro E. Sánchez-Jiménez , Nabil Amghar , Ricardo Chacartegui , José Manuel Valverde , Luis A. Pérez-Maqueda
|
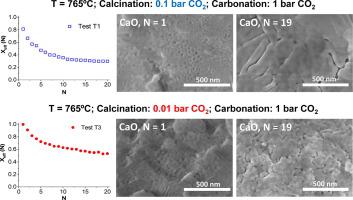
The Calcium Looping performance of limestone for thermochemical energy storage has been investigated under novel favorable conditions, which involve calcination at moderate temperatures under CO2 at low pressure (0.01 and 0.1 bar) and carbonation at high temperature under CO2 at atmospheric pressure. Calcining at low CO2 pressures allows to substantially reducing the temperature to achieve full calcination in short residence times. Moreover, it notably enhances CaO multicycle conversion. The highest values of conversion are obtained for limestone samples calcined under 0.01 bar CO2 at 765°C. Under these conditions, the residual conversion is increased by a factor of 10 as compared to conditions involving calcination under CO2 at atmospheric pressure. The enhancement of CaO conversion is correlated to the microstructure of the CaO samples obtained after calcination. As seen from SEM, BET surface and XRD analysis, calcination under low CO2 pressure leads to a remarkable decrease of pore volume and CaO crystallite size. Consequently, CaO surface area available for carbonation in the fast reaction-controlled regime and therefore reactivity in short residence times is promoted.
中文翻译:

在低CO 2压力下煅烧可增强石灰石用于热化学能量存储的钙循环性能
石灰石在热化学储能方面的钙环化性能已经在新的有利条件下进行了研究,包括在中等温度下于低压下在CO 2下煅烧(0.01和0.1 bar),在高温下于大气压下在CO 2下碳化。在低CO 2压力下煅烧可大大降低温度,从而在短停留时间内实现完全煅烧。此外,它显着增强了CaO多循环转化率。对于在0.01 bar CO 2和765°C下煅烧的石灰石样品,可获得最高的转化率。在这些条件下,与在CO下煅烧的条件相比,残余转化率提高了10倍2在大气压下。CaO转化率的提高与煅烧后获得的CaO样品的微观结构有关。从SEM,BET表面和XRD分析可以看出,在低CO 2压力下煅烧导致孔体积和CaO微晶尺寸显着减小。因此,促进了可在快速反应控制的条件下碳酸化的CaO表面积,因此促进了在短停留时间内的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号