Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-12-02 , DOI: 10.1016/j.bbamem.2020.183526 Irfan Prabudiansyah , Ramon van der Valk , Marie-Eve Aubin-Tam
|
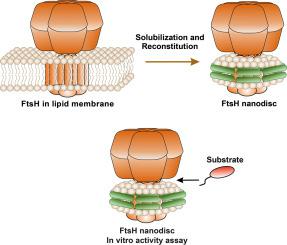
FtsH is a membrane-bound protease that plays a crucial role in proteolytic regulation of many cellular functions. It is universally conserved in bacteria and responsible for the degradation of misfolded or misassembled proteins. A recent study has determined the structure of bacterial FtsH in detergent micelles. To properly study the function of FtsH in a native-like environment, we reconstituted the FtsH complex into lipid nanodiscs. We found that FtsH in membrane scaffold protein (MSP) nanodiscs maintains its native hexameric conformation and is functionally active. We further investigated the effect of the lipid bilayer composition (acyl chain length, saturation, head group charge and size) on FtsH proteolytic activity. We found that the lipid acyl chain length influences AaFtsH activity in nanodiscs, with the greatest activity in a bilayer of di-C18:1 PC. We conclude that MSP nanodiscs are suitable model membranes for further in vitro studies of the FtsH protease complex.
中文翻译:

脂质纳米盘中FtsH蛋白酶的重构和功能表征
FtsH是膜结合的蛋白酶,在许多细胞功能的蛋白水解调节中起关键作用。它在细菌中普遍保守,并负责错误折叠或错误组装的蛋白质的降解。最近的一项研究确定了洗涤剂胶束中细菌FtsH的结构。为了正确研究FtsH在类似自然环境中的功能,我们将FtsH复合物重构为脂质纳米盘。我们发现膜支架蛋白(MSP)纳米光盘中的FtsH保持其天然六聚体构象并具有功能活性。我们进一步研究了脂质双层成分(酰基链长度,饱和度,头基电荷和大小)对FtsH蛋白水解活性的影响。我们发现脂质酰基链长度会影响纳米光盘中的AaFtsH活性,在di-C18:1 PC双层中具有最大的活性。我们得出的结论是,MSP纳米光盘是适合进一步应用的模型膜FtsH蛋白酶复合物的体外研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号