当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetics and Mechanisms of poly(N-isopropylacrylamide) Phase Transitions in Water–Methanol Solutions
Macromolecules ( IF 5.1 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.macromol.0c02253 Valerij Y. Grinberg 1, 2 , Tatiana V. Burova 1 , Natalia V. Grinberg 1 , Alexander P. Moskalets 1 , Alexander S. Dubovik 1, 2 , Irina G. Plashchina 2 , Alexei R. Khokhlov 1, 3
Macromolecules ( IF 5.1 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.macromol.0c02253 Valerij Y. Grinberg 1, 2 , Tatiana V. Burova 1 , Natalia V. Grinberg 1 , Alexander P. Moskalets 1 , Alexander S. Dubovik 1, 2 , Irina G. Plashchina 2 , Alexei R. Khokhlov 1, 3
Affiliation
|
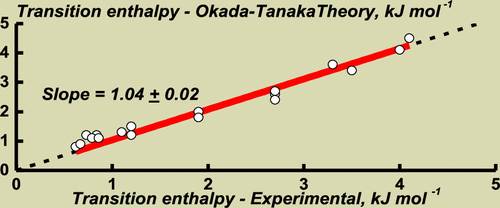
The phase transitions of poly(N-isopropylacrylamide) (PNIPAM) in water–methanol mixed solutions were studied in detail by high-sensitivity differential scanning calorimetry. From this study, the dependences of the transition temperature, enthalpy, heat capacity increment, and width on the methanol molar fraction (xMeOH) were obtained. The transition temperature passed through a minimum at the methanol molar fraction xMeOH* ∼ 0.35. At xMeOH < xMeOH*, the transition enthalpy decreased quickly with the methanol content and became so small that it could not be measured, even with an increase in the polymer concentration by a hundred times (up to 150 mg mL–1). Furthermore, over this xMeOH range, the transition heat capacity increment being negative remained practically constant, but the transition width sharply increased. The transition thermograms were quantitatively described by the Okada–Tanaka theory, which takes into account the role of the polymer–solvent interaction cooperativity in the polymer thermoresponsivity. In terms of this approach, it is assumed that over the defined range of methanol content, PNIPAM possesses the cooperative hydro-solvation structure in the form of water–methanol complexes. The energetics of this structure smoothly decreases with the increase in the methanol content up to a complete disappearance of the structure at xMeOH > xMeOH*. In this range of the methanol content, the phase behavior of PNIPAM seems to be dictated by regularities typical of polymer solutions in organic solvents, that is, how the Flory–Huggins parameter depends on temperature.
中文翻译:

水-甲醇溶液中聚(N-异丙基丙烯酰胺)相变的能级和机理
通过高灵敏度差示扫描量热法详细研究了聚(N-异丙基丙烯酰胺)(PNIPAM)在水-甲醇混合溶液中的相变。从这项研究中,获得了转变温度,焓,热容增量和宽度对甲醇摩尔分数(x MeOH)的依赖性。转变温度在甲醇的摩尔分数x MeOH *〜0.35时达到最小值。在x MeOH < x MeOH *时,过渡焓随甲醇含量而迅速降低,并且变小到无法测量,即使聚合物浓度增加了100倍(最高150 mg mL–1)。此外,在该x MeOH范围内,过渡热容增量为负值几乎保持恒定,但过渡宽度急剧增加。Okada-Tanaka理论定量描述了转变热分析图,该理论考虑了聚合物-溶剂相互作用协同性在聚合物热响应性中的作用。根据这种方法,假定在规定的甲醇含量范围内,PNIPAM具有水-甲醇配合物形式的协同加氢溶剂化结构。该结构的能级随着甲醇含量的增加而平稳地降低,直到在x MeOH > x MeOH下结构完全消失为止*。在此甲醇含量范围内,PNIPAM的相态行为似乎由有机溶剂中聚合物溶液的典型规律性决定,即,弗洛里-哈金斯参数如何取决于温度。
更新日期:2020-12-22
中文翻译:

水-甲醇溶液中聚(N-异丙基丙烯酰胺)相变的能级和机理
通过高灵敏度差示扫描量热法详细研究了聚(N-异丙基丙烯酰胺)(PNIPAM)在水-甲醇混合溶液中的相变。从这项研究中,获得了转变温度,焓,热容增量和宽度对甲醇摩尔分数(x MeOH)的依赖性。转变温度在甲醇的摩尔分数x MeOH *〜0.35时达到最小值。在x MeOH < x MeOH *时,过渡焓随甲醇含量而迅速降低,并且变小到无法测量,即使聚合物浓度增加了100倍(最高150 mg mL–1)。此外,在该x MeOH范围内,过渡热容增量为负值几乎保持恒定,但过渡宽度急剧增加。Okada-Tanaka理论定量描述了转变热分析图,该理论考虑了聚合物-溶剂相互作用协同性在聚合物热响应性中的作用。根据这种方法,假定在规定的甲醇含量范围内,PNIPAM具有水-甲醇配合物形式的协同加氢溶剂化结构。该结构的能级随着甲醇含量的增加而平稳地降低,直到在x MeOH > x MeOH下结构完全消失为止*。在此甲醇含量范围内,PNIPAM的相态行为似乎由有机溶剂中聚合物溶液的典型规律性决定,即,弗洛里-哈金斯参数如何取决于温度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号