当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization and Investigation of Hydrogen Evolution Activity of Ni‐Mo/Al2O3 Composite
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-12-01 , DOI: 10.1002/slct.202003889 V. Salarvand 1 , H. Mahmoodi 2 , F. Ahmadian 1 , N. Farahbakhsh 3 , A. Yousefifar 1 , M. Saghafi 1 , M. T. Noghani 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-12-01 , DOI: 10.1002/slct.202003889 V. Salarvand 1 , H. Mahmoodi 2 , F. Ahmadian 1 , N. Farahbakhsh 3 , A. Yousefifar 1 , M. Saghafi 1 , M. T. Noghani 1
Affiliation
|
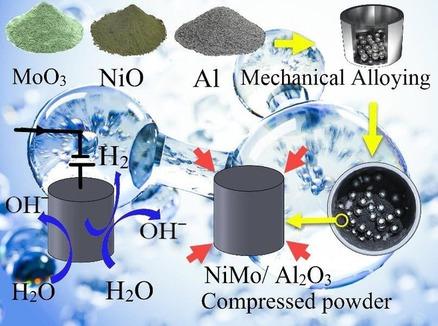
In the present study, the hydrogen evolution activity of the Ni−Mo/Al2O3 catalyst was investigated. This catalyst was produced by milling of oxide precursors of nickel oxide (NiO) and molybdenum trioxide (MoO3) with aluminum as a reducing agent. So that oxide precursors with 100 % excess aluminum on stoichiometric composition at different times were milled to perform complete reduction operations. Subsequently, X‐ray diffraction (XRD) analysis was performed for phase analysis and scanning electron microscopy (SEM) was used to investigate the microstructure. It was found that oxide precursors with 100 % excess aluminum on stoichiometric composition were reduced after 50 h of milling and nickel molybdenum/alumina composite was produced. On the other hand, to compare the activity of Ni−Mo/Al2O3 two different catalysts (57 NiMo, 80 NiMo) were also produced by the milling method. The specimens were transformed into pills of 10 mm in diameter and 3 mm in height at 900 MPa pressure. A furnace under an argon atmosphere at a temperature of 1400 °C was used to sinter the produced pills. Finally, the hydrogen evolution activity of this sample was investigated by linear polarization tests and impedance spectroscopy in 1 M KOH solution. The sample reduced with 100 % excess aluminum showed higher activity than the stoichiometric combination, with −62 mV cathodic‐Tafel slope, −70 mV over‐potential at a current density of −10 mA cm−2 and a charge transfer resistance of 126.6 ohms.
中文翻译:

Ni-Mo / Al2O3复合材料的合成,表征和析氢活性的研究
在本研究中,研究了Ni-Mo / Al 2 O 3催化剂的析氢活性。通过研磨氧化镍(NiO)和三氧化钼(MoO 3),以铝为还原剂。以便研磨在不同时间化学计量组成上具有100%过量铝的氧化物前体,以执行完全还原操作。随后,进行了X射线衍射(XRD)分析以进行相分析,并使用扫描电子显微镜(SEM)研究了显微结构。发现在研磨50小时后,具有在化学计量组成上过量的铝的100%的氧化物前体被还原并且产生了镍钼/氧化铝复合物。另一方面,比较Ni-Mo / Al 2 O 3的活性通过研磨方法还生产了两种不同的催化剂(57 NiMo,80 NiMo)。将样品在900 MPa压力下制成直径10毫米,高度3毫米的药丸。使用在氩气氛下在1400℃的温度下的炉来烧结所产生的丸剂。最后,通过线性极化试验和阻抗谱在1 M KOH溶液中研究了该样品的析氢活性。用100%过量的铝还原的样品显示出比化学计量的组合更高的活性,在-10 mA cm -2的电流密度下具有-62 mV的阴极-Tafel斜率,-70 mV的超电势和126.6 ohms的电荷转移电阻。
更新日期:2020-12-01
中文翻译:

Ni-Mo / Al2O3复合材料的合成,表征和析氢活性的研究
在本研究中,研究了Ni-Mo / Al 2 O 3催化剂的析氢活性。通过研磨氧化镍(NiO)和三氧化钼(MoO 3),以铝为还原剂。以便研磨在不同时间化学计量组成上具有100%过量铝的氧化物前体,以执行完全还原操作。随后,进行了X射线衍射(XRD)分析以进行相分析,并使用扫描电子显微镜(SEM)研究了显微结构。发现在研磨50小时后,具有在化学计量组成上过量的铝的100%的氧化物前体被还原并且产生了镍钼/氧化铝复合物。另一方面,比较Ni-Mo / Al 2 O 3的活性通过研磨方法还生产了两种不同的催化剂(57 NiMo,80 NiMo)。将样品在900 MPa压力下制成直径10毫米,高度3毫米的药丸。使用在氩气氛下在1400℃的温度下的炉来烧结所产生的丸剂。最后,通过线性极化试验和阻抗谱在1 M KOH溶液中研究了该样品的析氢活性。用100%过量的铝还原的样品显示出比化学计量的组合更高的活性,在-10 mA cm -2的电流密度下具有-62 mV的阴极-Tafel斜率,-70 mV的超电势和126.6 ohms的电荷转移电阻。











































 京公网安备 11010802027423号
京公网安备 11010802027423号