当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Three‐Component Coupling of Heteroarenes, Cycloalkenes and Propargylic Acetates
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-01 , DOI: 10.1002/anie.202014781 Shenghan Teng 1 , Yonggui Robin Chi 1 , Jianrong Steve Zhou 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-01 , DOI: 10.1002/anie.202014781 Shenghan Teng 1 , Yonggui Robin Chi 1 , Jianrong Steve Zhou 2
Affiliation
|
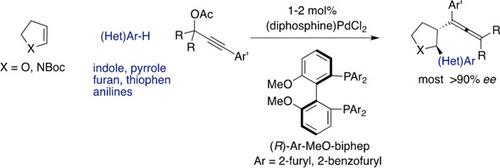
Asymmetric coupling proceeds efficiently between propargylic acetates, cycloalkenes and electron‐rich heteroarenes including indoles, pyrroles, activated furans and thiophenes. 2,3‐Disubstituted tetrahydrofurans and pyrrolidines are produced in trans configuration and excellent enantiomeric ratios. The reaction proceeds via Wacker‐type attack of nucleophilic heteroarenes on alkenes activated by allenyl PdII species.
中文翻译:

杂芳烃,环烯烃和炔丙基乙酸酯的对映选择性三组分偶联
炔丙基乙酸酯,环烯烃和富电子杂芳烃(包括吲哚,吡咯,活化的呋喃和噻吩)之间的不对称偶联有效地进行。2,3-二取代的四氢呋喃和吡咯烷以反式构型和出色的对映体比率生产。反应是通过瓦克型亲核杂芳烃对烯丙基Pd II物种活化的烯烃的攻击而进行的。
更新日期:2020-12-01
中文翻译:

杂芳烃,环烯烃和炔丙基乙酸酯的对映选择性三组分偶联
炔丙基乙酸酯,环烯烃和富电子杂芳烃(包括吲哚,吡咯,活化的呋喃和噻吩)之间的不对称偶联有效地进行。2,3-二取代的四氢呋喃和吡咯烷以反式构型和出色的对映体比率生产。反应是通过瓦克型亲核杂芳烃对烯丙基Pd II物种活化的烯烃的攻击而进行的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号