Structure ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.str.2020.11.008 Anne M Jecrois 1 , M Michael Dcona 2 , Xiaoyan Deng 3 , Dipankar Bandyopadhyay 4 , Steven R Grossman 5 , Celia A Schiffer 1 , William E Royer 1
|
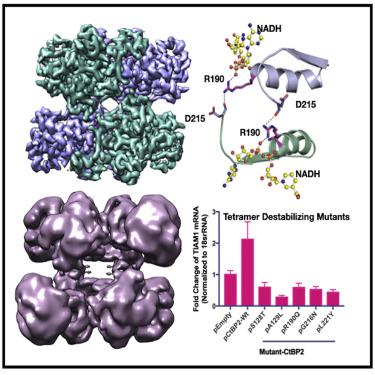
C-terminal binding proteins 1 and 2 (CtBP1 and CtBP2) are transcriptional regulators that activate or repress many genes involved in cellular development, apoptosis, and metastasis. NADH-dependent CtBP activation has been implicated in multiple types of cancer and poor patient prognosis. Central to understanding activation of CtBP in oncogenesis is uncovering how NADH triggers protein assembly, what level of assembly occurs, and if oncogenic activity depends upon such assembly. Here, we present the cryoelectron microscopic structures of two different constructs of CtBP2 corroborating that the native state of CtBP2 in the presence of NADH is tetrameric. The physiological relevance of the observed tetramer was demonstrated in cell culture, showing that CtBP tetramer-destabilizing mutants are defective for cell migration, transcriptional repression of E-cadherin, and activation of TIAM1. Together with our cryoelectron microscopy studies, these results highlight the tetramer as the functional oligomeric form of CtBP2.
中文翻译:

CtBP2 的冷冻电镜结构证实了四聚体结构
C 末端结合蛋白 1 和 2(CtBP1 和 CtBP2)是转录调节因子,可激活或抑制许多参与细胞发育、凋亡和转移的基因。NADH 依赖性 CtBP 激活与多种癌症和患者预后不良有关。了解 CtBP 在肿瘤发生中的激活的核心是揭示 NADH 如何触发蛋白质组装、发生的组装水平以及致癌活性是否取决于这种组装。在这里,我们展示了 CtBP2 的两种不同结构的低温电子显微镜结构,证实了在 NADH 存在下 CtBP2 的天然状态是四聚体。观察到的四聚体的生理相关性在细胞培养中得到证实,表明 CtBP 四聚体不稳定突变体对细胞迁移有缺陷,E-cadherin 的转录抑制和 TIAM1 的激活。连同我们的低温电子显微镜研究,这些结果突出了四聚体作为 CtBP2 的功能性寡聚形式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号