Structure ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.str.2020.11.006 Alex J Guseman 1 , Matthew J Whitley 1 , Jeremy J González 1 , Nityam Rathi 1 , Mikayla Ambarian 1 , Angela M Gronenborn 1
|
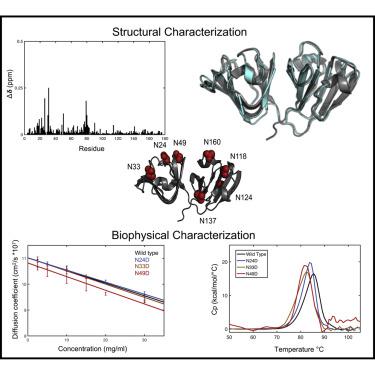
Cataracts involve the deposition of the crystallin proteins in the vertebrate eye lens, causing opacification and blindness. They are associated with either genetic mutation or protein damage that accumulates over the lifetime of the organism. Deamidation of Asn residues in several different crystallins has been observed and is frequently invoked as a cause of cataract. Here, we investigated the properties of Asp variants, deamidation products of γD-crystallin, by solution NMR, X-ray crystallography, and other biophysical techniques. No substantive structural or stability changes were noted for all seven Asn to Asp γD-crystallins. Importantly, no changes in diffusion interaction behavior could be detected. Our combined experimental results demonstrate that introduction of single Asp residues on the surface of γD-crystallin by deamidation is unlikely to be the driver of cataract formation in the eye lens.
中文翻译:

评估γD-结晶脱酰胺变体的结构和相互作用
白内障涉及晶状体蛋白在脊椎动物眼晶状体中的沉积,导致混浊和失明。它们与在生物体的一生中积累的基因突变或蛋白质损伤有关。已经观察到几种不同晶状体蛋白中 Asn 残基的脱酰胺,并且经常被认为是白内障的原因。在这里,我们通过溶液核磁共振、X 射线晶体学和其他生物物理技术研究了 Asp 变体、γD-晶状体蛋白的脱酰胺产物的特性。所有七种 Asn 到 Asp γD-晶状体蛋白都没有发现实质性的结构或稳定性变化。重要的是,没有检测到扩散相互作用行为的变化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号