当前位置:
X-MOL 学术
›
J. Phys. Chem. Solids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave-induced Selective Decomposition of Cellulose: Computational and Experimental Mechanistic Study
Journal of Physics and Chemistry of Solids ( IF 4.3 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jpcs.2020.109858 Duane D. Miller , Mark W. Smith , Dushyant Shekhawat
Journal of Physics and Chemistry of Solids ( IF 4.3 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jpcs.2020.109858 Duane D. Miller , Mark W. Smith , Dushyant Shekhawat
|
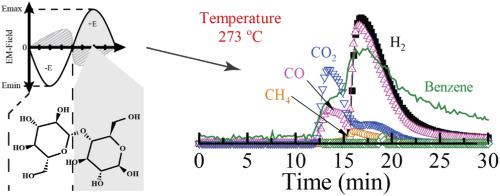
ABSTRACT Understanding microwave-material interactions will help facilitate the utilization of microwave technology in gasification and renewable energy production. In this study, cellulose was used as a model compound to simulate the organic matter in biomass, and its catalytic decomposition under a microwave (MW) field was studied to identify structural changes from the reaction. The study was conducted using a MW source coupled to a fixed-bed gas-flow reactor, mass spectroscopic, and Fourier transform infrared spectroscopy post-reaction analysis. Zeolite 13X was chosen as a microwave absorber to study the catalytic enhancement of the decomposition of cellulose. Density functional theory (DFT) was used to gain insights into the molecular transformations occurring in the presence of a static electric field, which was used to simulate the electric field component of the microwave electromagnetic radiation providing a theoretical basis for molecular sites to be selectively heated. Theoretical calculations demonstrated that both the positive and negative portion of the electric field interact with the permanent dipoles of the cellulose leading to Debye-type loss processes and localized heating indicated by the decomposition through the glycosidic bond breaking mechanism. The theoretical result was verified using infrared spectroscopic analysis of the pure cellulose during microwave heating. The theoretical calculations help to elucidate the dipoles and molecular bonds in the cellulose structure, which are more sensitive to selectively localized heating as compared to other bonds and conventional heating. Physically mixing Zeolite 13X with the cellulose led to a significant enhancement in the decomposition rate of the glycosidic bond. Zeolite 13X enhanced the glycosidic O-C decomposition at lower MW power (lower temperatures), whereas the O-H functional group required higher MW power (higher temperature) for its decomposition. The DFT study coupled with the reaction studies revealed that the electric field polarizability, and subsequent, localized heating, is dependent upon both the direction and the orientation of the cellulose. Gas products revealed that applying 250 W of MW power led to the production of CO, H2, along with some CO2, CH4, and benzene at 305 °C. Reaction under 500, 750, and 1000 W of power at constant temperature (305°C) revealed that higher power led to the complete decomposition of cellulose to mostly CO, H2, and CO2.
中文翻译:

微波诱导纤维素选择性分解:计算和实验机理研究
摘要 了解微波-材料相互作用将有助于促进微波技术在气化和可再生能源生产中的应用。在这项研究中,纤维素被用作模型化合物来模拟生物质中的有机物,并研究其在微波 (MW) 场下的催化分解,以识别反应中的结构变化。该研究使用与固定床气流反应器耦合的 MW 源、质谱和傅里叶变换红外光谱后反应分析进行。选择沸石 13X 作为微波吸收剂来研究纤维素分解的催化增强作用。密度泛函理论 (DFT) 用于深入了解在静电场存在下发生的分子转换,用于模拟微波电磁辐射的电场分量,为分子位点选择性加热提供理论基础。理论计算表明,电场的正负部分都与纤维素的永久偶极子相互作用,导致德拜型损失过程和通过糖苷键断裂机制分解所指示的局部加热。使用微波加热过程中纯纤维素的红外光谱分析验证了理论结果。理论计算有助于阐明纤维素结构中的偶极子和分子键,与其他键和常规加热相比,它们对选择性局部加热更敏感。将沸石 13X 与纤维素物理混合可显着提高糖苷键的分解速度。沸石 13X 在较低 MW 功率(较低温度)下增强了糖苷 OC 分解,而 OH 官能团需要较高的 MW 功率(较高温度)才能分解。结合反应研究的 DFT 研究表明,电场极化率和随后的局部加热取决于纤维素的方向和取向。气体产品显示,在 305 °C 时,应用 250 W MW 功率会导致产生 CO、H2 以及一些 CO2、CH4 和苯。在恒温 (305°C) 下,功率为 500、750 和 1000 W 的反应表明,更高的功率导致纤维素完全分解为主要是 CO、H2 和 CO2。
更新日期:2021-03-01
中文翻译:

微波诱导纤维素选择性分解:计算和实验机理研究
摘要 了解微波-材料相互作用将有助于促进微波技术在气化和可再生能源生产中的应用。在这项研究中,纤维素被用作模型化合物来模拟生物质中的有机物,并研究其在微波 (MW) 场下的催化分解,以识别反应中的结构变化。该研究使用与固定床气流反应器耦合的 MW 源、质谱和傅里叶变换红外光谱后反应分析进行。选择沸石 13X 作为微波吸收剂来研究纤维素分解的催化增强作用。密度泛函理论 (DFT) 用于深入了解在静电场存在下发生的分子转换,用于模拟微波电磁辐射的电场分量,为分子位点选择性加热提供理论基础。理论计算表明,电场的正负部分都与纤维素的永久偶极子相互作用,导致德拜型损失过程和通过糖苷键断裂机制分解所指示的局部加热。使用微波加热过程中纯纤维素的红外光谱分析验证了理论结果。理论计算有助于阐明纤维素结构中的偶极子和分子键,与其他键和常规加热相比,它们对选择性局部加热更敏感。将沸石 13X 与纤维素物理混合可显着提高糖苷键的分解速度。沸石 13X 在较低 MW 功率(较低温度)下增强了糖苷 OC 分解,而 OH 官能团需要较高的 MW 功率(较高温度)才能分解。结合反应研究的 DFT 研究表明,电场极化率和随后的局部加热取决于纤维素的方向和取向。气体产品显示,在 305 °C 时,应用 250 W MW 功率会导致产生 CO、H2 以及一些 CO2、CH4 和苯。在恒温 (305°C) 下,功率为 500、750 和 1000 W 的反应表明,更高的功率导致纤维素完全分解为主要是 CO、H2 和 CO2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号