Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2020-11-29 , DOI: 10.1016/j.jorganchem.2020.121642 Sonam Verma , Gulzar A. Bhat , Ramaswamy Murugavel
|
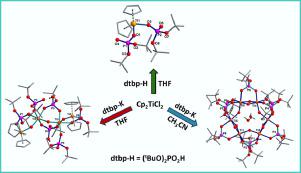
Reaction of titanocene dichloride [Cp2TiCl2] (Cp = C5H5) with (tBuO)2PO2H (di-tert-butylphosphate, dtbpH) in THF at room temperature results in the formation of mononuclear titanophosphate [Cp2Ti(dtbp)2].H2O (1), whereas the reaction of potassium salt of dtbpH (dtbpK) with [Cp2TiCl2] in THF yields tetranuclear cluster [Cp4Ti4(µ-O)3(µ-dtbp)2(dtbp)2(µ-mtbpH)2].2C6H5CH3 (2) (mtbpH = (tBuO)PO3H). Change of the reaction medium to acetonitrile for the reaction dtbpK with [Cp2TiCl2] leads to the isolation of novel hexanuclear titanophosphate cluster [Ti6(µ-O)6(µ-OH)3(µ-dtbp)9] (3), which is free of cyclopentadienyl ligands. Compound 1, when dissolved in CH3CN and allowed to stand under aerobic conditions, converts to either 2 or 3, depending on the dilution (water content in the medium) and the duration of crystallization. While lesser water content and shorter crystallization periods produce 2 as orange crystals, longer exposure of the solution results in the complete hydrolysis of Cp-Ti linkages and produce colorless, Cp-free hexanuclear cluster 3. In contrary, the reaction of either dtbpH or its potassium salt with zirconocene dichloride exclusively leads to the formation of [CpZr(µ-OH)(µ-dtbp)(dtbp)]2 (4) as the only product. Compounds 1-4 have been characterized by analytical and spectroscopic methods. Their molecular structures have further been established by single-crystal X-ray diffraction studies. Thermal decomposition of 1 reveals its thermolabile nature with the initial loss of alkyl substituents of the organophosphate ligands through β-hydride elimination followed by the loss of C5H5 groups to form an organic-free crystalline titanium phosphate phases which sinter finally to titanium pyrophosphate TiP2O7 at 800 ˚C.
中文翻译:

来源于茂金属二氯化物的不耐热钛和锆有机磷酸盐中环戊二烯基去除的辅助核扩展。
室温下二茂钛二氯化物[Cp 2 TiCl 2 ](Cp = C 5 H 5)与(t BuO)2 PO 2 H(磷酸二叔丁酯,dtbpH)在THF中的反应导致形成单核钛磷酸盐[Cp 2 Ti(dtbp)2 ] .H 2 O(1),而dtbpH的钾盐(dtbpK)与[Cp 2 TiCl 2 ]在THF中的反应生成四核簇[Cp 4 Ti 4(µ-O)3( µ-dtbp)2(dtbp)2(μ-mtbpH)2 ] .2C 6 H 5 CH 3(2)(mtbpH =(t BuO)PO 3 H)。将dtbpK与[Cp 2 TiCl 2 ]反应的反应介质更改为乙腈可导致分离出新的六核钛磷酸盐簇[Ti 6(µ-O)6(µ-OH)3(µ-dtbp)9 ](3),其不含环戊二烯基配体。化合物1溶于CH 3 CN并在有氧条件下放置时,会转化为2或3,具体取决于稀释度(介质中的水含量)和结晶时间。较少的水含量和较短的结晶时间会生成2个橙色晶体,而溶液的长时间暴露会导致Cp-Ti键完全水解,并产生无色,不含Cp的六核簇3。相反,dtbpH或其反应钾盐与二氧化锆一起唯一地导致形成[CpZr(µ-OH)(µ-dtbp)(dtbp)] 2(4)。化合物1-4已经通过分析和光谱方法表征。通过单晶X射线衍射研究进一步确定了它们的分子结构。1的热分解显示了其热不稳定的性质,有机磷配体的烷基取代基最初通过β氢化物消除而失去,然后失去C 5 H 5基团形成无有机的结晶磷酸钛相,最终烧结成焦磷酸钛。 TiP 2 O 7在800˚C 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号