Comparative Biochemistry and Physiology A: Molecular & Integrative Physiology ( IF 2.1 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.cbpa.2020.110855 Till S Harter 1 , Colin J Brauner 2
|
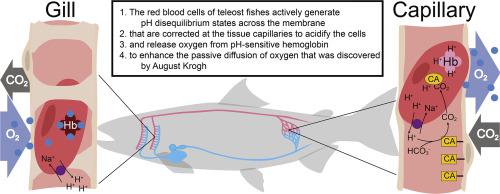
In the early 20th century, August and Marie Krogh settled one of the most controversial questions in physiology, showing through elegant experiments that oxygen (O2) uptake at the lung is driven by passive diffusion alone. Krogh's later work, on the regulation of local blood flow and capillary recruitment at the tissues, was awarded with the Nobel Prize in 1920. A century later it is still undisputed that O2 moves across tissues by diffusion, however, animals use active mechanisms to regulate and facilitate the passive process. Teleost fishes have evolved a mechanism by which adrenergic sodium-proton-exchangers (β-NHEs) on the red blood cell (RBC) membrane actively create H+ gradients that are short-circuited in the presence of plasma-accessible carbonic anhydrase (CA) at the tissue capillaries. The rapid acidification of the RBC reduces the O2 affinity of pH-sensitive haemoglobin, which increases the O2 diffusion gradient to the tissues. When RBCs leave the site of plasma-accessible CA, β-NHE activity recovers RBC pH during venous transit, to promote renewed O2 loading at the gills. This mechanism allows teleosts to unload more O2 at their tissues without compromising O2 diffusion gradients and therefore, to use the available O2 carrying capacity of the blood to a greater degree. In Atlantic salmon, β-NHE short-circuiting reduces the requirements on the heart by up to 30% during moderate exercise and even at rest, with important ecological implications. Thus, in some teleosts, the RBCs participate in regulating the systemic O2 flux by actively altering the passive diffusion of O2 that Krogh discovered.
中文翻译:

硬骨红细胞主动增强了奥古斯特·克罗 (August Krogh) 发现的氧气被动扩散
在 20 世纪初期,August 和 Marie Krogh 解决了生理学中最具争议的问题之一,通过优雅的实验表明,肺的氧气 (O 2 ) 摄取仅由被动扩散驱动。克罗的后期工作,对局部血流量和毛细管招聘在组织的监管,与诺贝尔文学奖颁发于1920年。一个世纪后,它仍然是无可争议的Ø 2跨组织移动通过扩散,但是,动物使用积极的机制规范和促进被动过程。硬骨鱼进化出一种机制,通过该机制,红细胞 (RBC) 膜上的肾上腺素钠质子交换剂 (β-NHE) 主动产生 H +在组织毛细血管处存在血浆可及的碳酸酐酶 (CA) 时,梯度会被短路。红细胞的快速酸化降低了对 pH 值敏感的血红蛋白的 O 2亲和力,从而增加了 O 2向组织的扩散梯度。当红细胞离开血浆可及 CA 的部位时,β-NHE 活性会在静脉转运过程中恢复红细胞的 pH,以促进鳃处重新加载O 2。这种机制允许硬骨鱼在其组织中卸载更多的 O 2而不影响 O 2扩散梯度,因此,使用可用的 O 2血液的承载能力更大。在大西洋鲑鱼中,β-NHE 短路可使适度运动甚至休息时对心脏的需求降低多达 30%,具有重要的生态意义。因此,在一些硬骨鱼中,红细胞通过主动改变Krogh 发现的 O 2的被动扩散来参与调节全身 O 2通量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号