Chemosphere ( IF 8.1 ) Pub Date : 2020-11-29 , DOI: 10.1016/j.chemosphere.2020.129079 Lujian Lin , Shuai Tang , Xuesong Wang , Xuan Sun , Anqi Yu
|
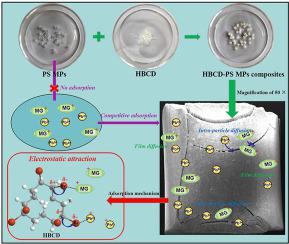
The role of microplastics (MPs) as a carrier of pollutants in water environment is an emerging issue; however, information regarding the underlying mechanisms for malachite green (MG) and Pb(II) adsorption onto hexabromocyclododecane (HBCD)-polystyrene (PS) composites MPs (HBCD-PS MPs) is still lacking. In this study, the adsorption behaviors and mechanisms of MG and Pb(II) onto PS and HBCD-PS MPs were investigated in batch adsorption experiments. The amounts of MG and Pb(II) adsorbed onto PS MPs were negligible while the presence of HBCD significantly enhanced the adsorption of MG and Pb(II) onto HBCD-PS MPs. The results of intra-particle and film diffusion model confirmed that the adsorption of MG and Pb(II) onto HBCD-PS MPs was dominated by intra-particle diffusion. The maximum adsorption amount of Pb(II) and MG onto HBCD-PS MPs followed the sequence of Pb(II) (3.33 μmol g-1) > MG (1.87 μmol g-1). In binary systems, MG and Pb(II) showed competitive adsorption onto HBCD-PS MPs, and Pb(II) exhibited relatively higher affinity to be adsorbed onto HBCD-PS MPs. Solution pH and salinity played a crucial role in the adsorption process. XPS analysis suggested that the –Br participated in the adsorption process as an electron-withdrawing group. Overall, electrostatic interaction regulated the adsorption of MG and Pb(II) onto HBCD-PS MPs. Results from this study demonstrated that HBCD could enhance the role of MPs in the MG and Pb(II) migration by changing their adsorption behavior onto MPs.
中文翻译:

六溴环十二烷改变孔雀石绿和铅在聚苯乙烯微塑料上的吸附行为:相互作用机理和竞争效应
微塑料(MPs)在水环境中作为污染物载体的作用是一个新兴的问题;但是,仍然缺少有关孔雀石绿(MG)和Pb(II)吸附到六溴环十二烷(HBCD)-聚苯乙烯(PS)复合材料MP(HBCD-PS MP)上的潜在机理的信息。在本研究中,通过分批吸附实验研究了MG和Pb(II)在PS和HBCD-PS MPs上的吸附行为和机理。吸附在PS MP上的MG和Pb(II)的量可以忽略不计,而HBCD的存在显着增强了MG和Pb(II)在HBCD-PS MP上的吸附。颗粒内和膜扩散模型的结果证实,MGCD和Pb(II)在HBCD-PS MPs上的吸附主要是颗粒内扩散。最大吸附量将Pb(II)和MG置于HBCD-PS MP上的顺序为Pb(II)(3.33μmolg -1)> MG(1.87μmolg -1)。在二元体系中,MG和Pb(II)在HBCD-PS MPs上显示出竞争性吸附,而Pb(II)在HBCD-PS MPs上显示出相对较高的亲和力。溶液的pH和盐度在吸附过程中起着至关重要的作用。XPS分析表明,-Br作为吸电子基团参与了吸附过程。总体而言,静电相互作用调节了MGCD和Pb(II)在HBCD-PS MPs上的吸附。这项研究的结果表明,六溴环十二烷可以通过改变MPs对MPs的吸附行为来增强MPs在MG和Pb(II)迁移中的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号