当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Pictet-Spengler cyclisations through acceptorless iridium-catalysed dehydrogenation of tertiary amines
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-30 , DOI: 10.1016/j.tet.2020.131785 John P. Cooksey , Ourida Saidi , Jonathan M.J. Williams , A. John Blacker , Stephen P. Marsden
中文翻译:

通过无受体铱催化的叔胺脱氢氧化Pictet-Spengler环化反应
更新日期:2020-12-17
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-30 , DOI: 10.1016/j.tet.2020.131785 John P. Cooksey , Ourida Saidi , Jonathan M.J. Williams , A. John Blacker , Stephen P. Marsden
|
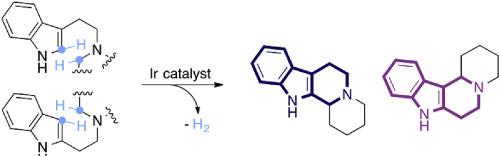
The valuable tetrahydro-β- and γ-carboline skeleta can be accessed through Pictet-Spengler cyclisation initiated by acceptorless dehydrogenation of saturated cyclic amines. The substrate scope for the β-isomers is found to be somewhat limited, but access to the γ-isomers through the more reactive 2-(aminoethyl)indoles is more general. The synthetic utility of hydrogen transfer catalysis is highlighted in a two-step preparation of the alkaloid desbromoarborescidine A by sequential redox-neutral alkylation/dehydrogenative cyclisation.
中文翻译:

通过无受体铱催化的叔胺脱氢氧化Pictet-Spengler环化反应
有价值的四氢-β-和γ-咔啉骨架可以通过Pictet-Spengler环化反应获得,该环化反应由饱和环胺的无受体脱氢反应引发。发现β-异构体的底物范围受到一定限制,但是通过反应性更强的2-(氨乙基)吲哚接近γ-异构体的途径更为普遍。通过顺序的氧化还原-中性烷基化/脱氢环化反应,生物碱去溴阿糖胞苷A的两步制备突出了氢转移催化的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号