当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic asymmetric total syntheses of (R)-bgugaine and (R)-irnidine
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.tet.2020.131789 Christopher J. Maddocks , Paul A. Clarke
中文翻译:

(R)-双胍丁胺和(R)-乙啶的催化不对称全合成
更新日期:2020-12-17
Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.tet.2020.131789 Christopher J. Maddocks , Paul A. Clarke
|
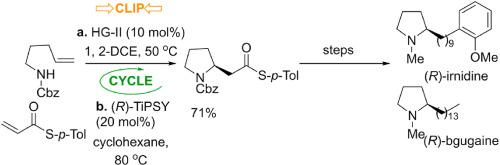
An enantioselective total synthesis of (R)-bgugaine and the first enantioselective total synthesis of (R)-irnidine are reported. The key steps are the asymmetric ‘clip-cycle’ formation of the pyrrolidine ring in 94:6 e.r., which is common to both natural products, followed by Liebeskind–Srogl coupling and Wolf-Kishner reduction. The route yields (R)-bgugaine and (R)-irnidine in 6 steps and in overall yields of 33% and 18% respectively.
中文翻译:

(R)-双胍丁胺和(R)-乙啶的催化不对称全合成
的对映体选择性全合成(- [R)-bgugaine和(的第一对映选择性全合成- [R)-irnidine报告。关键步骤是在94:6 er中吡咯烷环的不对称“剪切循环”形成,这对两种天然产物都是常见的,随后是Liebeskind-Srogl偶联和Wolf-Kishner还原。该路线分6步产生(R)-古吉ine碱和(R)-亚氨酸idine,总产率分别为33%和18%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号