Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.yjmcc.2020.11.009 Haifeng Yin 1 , Amanda J Favreau-Lessard 1 , Joanne T deKay 1 , Yodit R Herrmann 1 , Michael P Robich 2 , Robert A Koza 1 , Igor Prudovsky 1 , Douglas B Sawyer 2 , Sergey Ryzhov 1
|
Background
Myeloid cells play an important role in a wide variety of cardiovascular disorders, including both ischemic and non-ischemic cardiomyopathies. Neuregulin-1 (NRG-1)/ErbB signaling has recently emerged as an important factor contributing to the control of inflammatory activation of myeloid cells after an ischemic injury. However, the role of ErbB signaling in myeloid cells in non-ischemic cardiomyopathy is not fully understood. This study investigated the role of ErbB3 receptors in the regulation of early adaptive response using a mouse model of transverse aortic constriction (TAC) for non-ischemic cardiomyopathy.
Methods and results
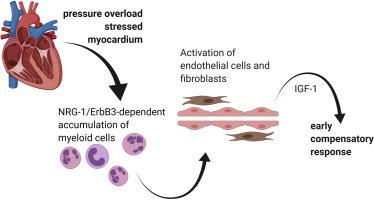
TAC surgery was performed in groups of age- and sex-matched myeloid cell-specific ErbB3-deficient mice (ErbB3MyeKO) and control animals (ErbB3MyeWT). The number of cardiac CD45 immune cells, CD11b myeloid cells, Ly6G neutrophils, and Ly6C monocytes was determined using flow cytometric analysis. Five days after TAC, survival was dramatically reduced in male but not female ErbB3MyeKO mice or control animals. The examination of lung weight to body weight ratio suggested that acute pulmonary edema was present in ErbB3MyeKO male mice after TAC. To determine the cellular and molecular mechanisms involved in the increased mortality in ErbB3MyeKO male mice, cardiac cell populations were examined at day 3 post-TAC using flow cytometry. Myeloid cells accumulated in control but not in ErbB3MyeKO male mouse hearts. This was accompanied by increased proliferation of Sca-1 positive non-immune cells (endothelial cells and fibroblasts) in control but not ErbB3MyeKO male mice. No significant differences in intramyocardial accumulation of myeloid cells or proliferation of Sca-1 cells were found between the groups of ErbB3MyeKO and ErbB3MyeWT female mice. An antibody-based protein array analysis revealed that IGF-1 expression was significantly downregulated only in ErbB3MyeKO mice hearts compared to control animals after TAC.
Conclusion
Our data demonstrate the crucial role of myeloid cell-specific ErbB3 signaling in the cardiac accumulation of myeloid cells, which contributes to the activation of cardiac endothelial cells and fibroblasts and development of an early adaptive response to cardiac pressure overload in male mice.
中文翻译:

ErbB3信号在骨髓细胞适应心脏压力超负荷过程中的保护作用
背景
骨髓细胞在多种心血管疾病中发挥重要作用,包括缺血性和非缺血性心肌病。Neuregulin-1 (NRG-1)/ErbB 信号转导最近已成为有助于控制缺血性损伤后骨髓细胞炎症激活的重要因素。然而,ErbB 信号在非缺血性心肌病骨髓细胞中的作用尚不完全清楚。本研究使用非缺血性心肌病横断主动脉缩窄 (TAC) 小鼠模型研究了 ErbB3 受体在调节早期适应性反应中的作用。
方法和结果
在年龄和性别匹配的骨髓细胞特异性 ErbB3 缺陷小鼠 (ErbB3 MyeKO ) 和对照动物 (ErbB3 MyeWT ) 组中进行 TAC 手术。使用流式细胞仪分析确定心脏 CD45 免疫细胞、CD11b 骨髓细胞、Ly6G 中性粒细胞和 Ly6C 单核细胞的数量。TAC 后五天,雄性但雌性 ErbB3 MyeKO小鼠或对照动物的存活率显着降低。肺重量与体重比的检查表明,TAC 后 ErbB3 MyeKO雄性小鼠出现急性肺水肿。确定 ErbB3 MyeKO死亡率增加所涉及的细胞和分子机制雄性小鼠,在 TAC 后第 3 天使用流式细胞术检查心脏细胞群。骨髓细胞在对照中积累,但在 ErbB3 MyeKO雄性小鼠心脏中没有积累。这伴随着对照而非 ErbB3 MyeKO雄性小鼠中 Sca-1 阳性非免疫细胞(内皮细胞和成纤维细胞)的增殖增加。在 ErbB3 MyeKO和 ErbB3 MyeWT雌性小鼠组之间,未发现骨髓细胞的心肌内积累或 Sca-1 细胞的增殖有显着差异。基于抗体的蛋白质阵列分析显示,与 TAC 后的对照动物相比,IGF-1 表达仅在 ErbB3 MyeKO小鼠心脏中显着下调。
结论
我们的数据证明了髓细胞特异性 ErbB3 信号传导在髓细胞心脏积累中的关键作用,这有助于激活心脏内皮细胞和成纤维细胞,并在雄性小鼠中形成对心脏压力超负荷的早期适应性反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号