Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-11-30 , DOI: 10.1016/j.jhazmat.2020.124658 Daniel Sanchez Carretero , Chih-pin Huang , Jing-Hua Tzeng , Chin-pao Huang
|
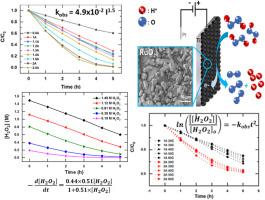
Piranha solution is a highly acidic mixture of sulfuric acid and hydrogen peroxide. The present study aimed at developing a dimensionally stable anode (DSA), made of titanium metal foil coated with Ruthenium Dioxide (RuO2), for the electrochemical oxidation of hydrogen peroxide in the presence of strong sulfuric acid under ambient conditions. Results showed that hydrogen peroxide in the piranha solution was fully degraded in 5 h under a constant current of 2 A (or current density of 0.32 A-cm-2).The oxidation kinetics of hydrogen peroxide followed the Langmuir-Hinshelwood model. The observed rate constant was a function of applied current. The initial current efficiency was 17.5% at 0.5 A (or 0.08 A-cm-2) and slightly decreased to about 13.5% at applied current between 1.3 and 1.5 A (or current density of 0.208 and 0.24 A-cm-2). Results showed the capability and feasibility of the electrochemical oxidation process for the recovery of sulfuric acid from the spent piranha solution in semiconductor industrial installations or general laboratories.
中文翻译:

尺寸稳定阳极(DSA)Ti-RuO 2电极上从耗竭的食人鱼溶液中回收硫酸
食人鱼溶液是硫酸和过氧化氢的高酸性混合物。本研究旨在开发一种尺寸稳定的阳极(DSA),该阳极由涂有二氧化钌(RuO 2)的钛金属箔制成,用于在环境条件下在强硫酸存在下对过氧化氢进行电化学氧化。结果表明,食人鱼溶液中的过氧化氢在2 A的恒定电流下(或电流密度为0.32 A-cm -2)在5小时内被完全降解。过氧化氢的氧化动力学遵循Langmuir-Hinshelwood模型。观察到的速率常数是施加电流的函数。在0.5 A(或0.08 A-cm -2)下的初始电流效率为17.5%),并且在1.3至1.5 A(或0.208至0.24 A-cm -2的电流密度)之间施加的电流下略微降低至约13.5%。结果表明,在半导体工业设施或一般实验室中,电化学氧化工艺可从食人鱼的废溶液中回收硫酸的能力和可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号