当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surfactant Impact on Interfacial Protein Aggregation and Utilization of Surface Tension to Predict Surfactant Requirements for Biological Formulations
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.molpharmaceut.0c00743 Kevin B Vargo 1 , Patrick Stahl 1 , Brian Hwang 1 , Erica Hwang 1 , Daniel Giordano 1 , Peyton Randolph 1 , Christina Celentano 1 , Robert Hepler 2 , Ketan Amin 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.molpharmaceut.0c00743 Kevin B Vargo 1 , Patrick Stahl 1 , Brian Hwang 1 , Erica Hwang 1 , Daniel Giordano 1 , Peyton Randolph 1 , Christina Celentano 1 , Robert Hepler 2 , Ketan Amin 1
Affiliation
|
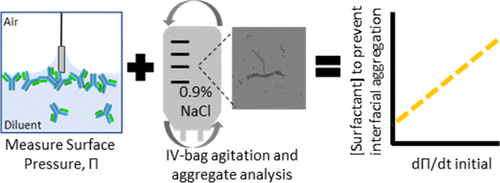
Biological drug products are formulated with excipients to maintain stability over the shelf life of the product. Surfactants are added to the drug product to stabilize air–water interfaces known to induce protein aggregation. Early formulation development is focused on maintaining protein conformation and colloidal stability over the course of the drug product shelf life but rarely considers stability through dose preparation and administration. Specifically, intravenous (IV) bag preparation exposes the therapeutic protein to a different solution environment concurrently diluting the stabilizing excipients that had been added to the drug product formulation. Mixing in IV bags can generate dynamic changes in the air–water interfacial area known to cause protein aggregation if not sufficiently protected. Therefore, understanding the surfactant requirements for drug product end-to-end stability in early formulation development provides critical information for a right-first-time approach to drug product formulation and robust clinical preparation. The goal of these studies was to understand if interfacial properties of proteins could predict surfactant formulation requirements for end-to-end stability. Specifically, the interfacial properties of five proteins were measured in 0.9% saline and 5% dextrose. Furthermore, shaking studies were conducted to identify the minimum surfactant concentration required to prevent subvisible and visible particle formulation in each diluent. The impact of surfactant type and concentration on particle generation and size was explored. A mathematical model was generated to predict the minimum surfactant concentration required to prevent interface-driven aggregation in each diluent based on the change in surface pressure upon exposure of the protein to the interface. The model was tested under typical IV-preparation conditions with experimental output closely matching the model prediction. By employing this model and better understanding the role of surfactants in interfacial stability, drug product development can generate robust end-to-end large molecule formulations across shelf life, dose preparation, and administration.
中文翻译:

表面活性剂对界面蛋白质聚集的影响和利用表面张力预测生物制剂的表面活性剂需求
生物药物产品与赋形剂一起配制,以在产品的保质期内保持稳定性。将表面活性剂添加到药物产品中以稳定已知会诱导蛋白质聚集的空气-水界面。早期的制剂开发侧重于在药品保质期内保持蛋白质构象和胶体稳定性,但很少考虑通过剂量制备和给药的稳定性。具体而言,静脉 (IV) 袋制剂将治疗性蛋白质暴露于不同的溶液环境,同时稀释已添加到药品制剂中的稳定赋形剂。混合在静脉输液袋中会导致空气-水界面区域发生动态变化,如果没有得到充分保护,已知会导致蛋白质聚集。所以,了解早期制剂开发中药物产品端到端稳定性的表面活性剂要求为药物产品制剂和稳健的临床制备的首次正确方法提供了关键信息。这些研究的目的是了解蛋白质的界面特性是否可以预测表面活性剂配方对端到端稳定性的要求。具体而言,在 0.9% 盐水和 5% 葡萄糖中测量了五种蛋白质的界面特性。此外,还进行了摇动研究以确定在每种稀释剂中防止不可见和可见颗粒配方所需的最小表面活性剂浓度。探讨了表面活性剂类型和浓度对颗粒生成和尺寸的影响。基于蛋白质暴露于界面时的表面压力变化,生成数学模型以预测防止每种稀释剂中界面驱动聚集所需的最小表面活性剂浓度。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地了解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地了解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地理解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。
更新日期:2021-01-04
中文翻译:

表面活性剂对界面蛋白质聚集的影响和利用表面张力预测生物制剂的表面活性剂需求
生物药物产品与赋形剂一起配制,以在产品的保质期内保持稳定性。将表面活性剂添加到药物产品中以稳定已知会诱导蛋白质聚集的空气-水界面。早期的制剂开发侧重于在药品保质期内保持蛋白质构象和胶体稳定性,但很少考虑通过剂量制备和给药的稳定性。具体而言,静脉 (IV) 袋制剂将治疗性蛋白质暴露于不同的溶液环境,同时稀释已添加到药品制剂中的稳定赋形剂。混合在静脉输液袋中会导致空气-水界面区域发生动态变化,如果没有得到充分保护,已知会导致蛋白质聚集。所以,了解早期制剂开发中药物产品端到端稳定性的表面活性剂要求为药物产品制剂和稳健的临床制备的首次正确方法提供了关键信息。这些研究的目的是了解蛋白质的界面特性是否可以预测表面活性剂配方对端到端稳定性的要求。具体而言,在 0.9% 盐水和 5% 葡萄糖中测量了五种蛋白质的界面特性。此外,还进行了摇动研究以确定在每种稀释剂中防止不可见和可见颗粒配方所需的最小表面活性剂浓度。探讨了表面活性剂类型和浓度对颗粒生成和尺寸的影响。基于蛋白质暴露于界面时的表面压力变化,生成数学模型以预测防止每种稀释剂中界面驱动聚集所需的最小表面活性剂浓度。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地了解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地了解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。该模型在典型的 IV 制备条件下进行了测试,实验输出与模型预测非常匹配。通过采用该模型并更好地理解表面活性剂在界面稳定性中的作用,药物产品开发可以在保质期、剂量制备和给药期间生成稳健的端到端大分子制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号