当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective N1/N4 1,4-Cycloaddition of 1,2,4,5-Tetrazines Enabled by Solvent Hydrogen Bonding
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-30 , DOI: 10.1021/jacs.0c09775 Zixi Zhu 1 , Christopher M Glinkerman 1 , Dale L Boger 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-30 , DOI: 10.1021/jacs.0c09775 Zixi Zhu 1 , Christopher M Glinkerman 1 , Dale L Boger 1
Affiliation
|
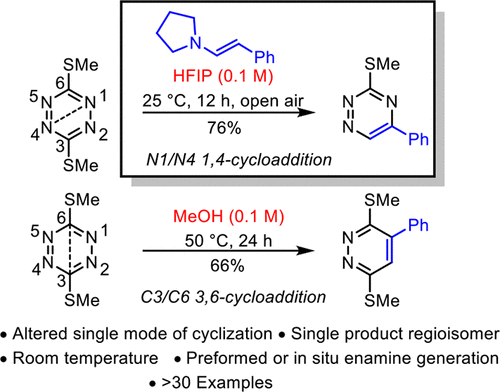
An unprecedented 1,4-cycloaddition (vs 3,6-cycloaddition) of 1,2,4,5-tetrazines is described with preformed or in situ generated aryl-conjugated enamines promoted by the solvent hydrogen bonding of hexafluoroisopropanol (HFIP) that is conducted under mild reaction conditions (0.1 M HFIP, 25 °C, 12 h). The reaction constitutes a formal [4 + 2] cycloaddition across the two nitrogen atoms (N1/N4) of the 1,2,4,5-tetrazine followed by a formal retro [4 + 2] cycloaddition loss of a nitrile and aromatization to generate a 1,2,4-triazine derivative. The factors that impact the remarkable change in the reaction mode, optimization of reaction parameters, the scope and simplification of its implementation through in situ enamine generation from aldehydes and ketones, the reaction scope for 3,6-bis(thiomethyl)-1,2,4,5-tetrazine, a survey of participating 1,2,4,5-tetrazines, and key mechanistic insights into this reaction are detailed. Given its simplicity and breath, the study establishes a novel method for the simple and efficient one-step synthesis of 1,2,4-triazines under mild conditions from readily accessible starting materials. Whereas alternative protic solvents (e.g., MeOH vs HFIP) provide products of the conventional 3,6-cycoladdition, the enhanced hydrogen bonding capability of HFIP uniquely results in promotion of the unprecedented formal 1,4-cycloaddition. As such, the studies represent an example of not just an enhancement in the rate or efficiency of a heterocyclic azadiene cycloaddition by hydrogen bonding catalysis but also the first to alter the mode (N1/N4 vs C3/C6) of cycloaddition.
中文翻译:

通过溶剂氢键实现 1,2,4,5-四嗪的选择性 N1/N4 1,4-环加成
描述了通过六氟异丙醇 (HFIP) 的溶剂氢键促进的预形成或原位生成的芳基共轭烯胺,发生了前所未有的 1,2,4,5-四嗪的 1,4-环加成(相对于 3,6-环加成),即在温和的反应条件下进行(0.1 M HFIP,25°C,12 小时)。该反应构成了 1,2,4,5-四嗪的两个氮原子 (N1/N4) 的正式 [4 + 2] 环加成,然后是腈的正式逆 [4 + 2] 环加成损失和芳构化生成1,2,4-三嗪衍生物。影响反应模式显着变化的因素、反应参数的优化、醛酮原位生成烯胺实施的范围和简化、3,6-双(硫甲基)-1,2的反应范围,4,5-四嗪,对参与的 1,2,4,5-四嗪的调查以及对该反应的关键机制的见解进行了详细介绍。鉴于其简单性和呼吸性,该研究建立了一种在温和条件下从容易获得的起始材料简单有效一步合成 1,2,4-三嗪的新方法。尽管替代质子溶剂(例如,MeOH 与 HFIP)提供了传统的 3,6-环加成产物,但 HFIP 增强的氢键合能力独特地促进了前所未有的正式 1,4-环加成。因此,这些研究不仅代表了通过氢键催化提高杂环氮杂二烯环加成速率或效率的例子,而且也是第一个改变环加成模式(N1/N4 与 C3/C6)的例子。
更新日期:2020-11-30
中文翻译:

通过溶剂氢键实现 1,2,4,5-四嗪的选择性 N1/N4 1,4-环加成
描述了通过六氟异丙醇 (HFIP) 的溶剂氢键促进的预形成或原位生成的芳基共轭烯胺,发生了前所未有的 1,2,4,5-四嗪的 1,4-环加成(相对于 3,6-环加成),即在温和的反应条件下进行(0.1 M HFIP,25°C,12 小时)。该反应构成了 1,2,4,5-四嗪的两个氮原子 (N1/N4) 的正式 [4 + 2] 环加成,然后是腈的正式逆 [4 + 2] 环加成损失和芳构化生成1,2,4-三嗪衍生物。影响反应模式显着变化的因素、反应参数的优化、醛酮原位生成烯胺实施的范围和简化、3,6-双(硫甲基)-1,2的反应范围,4,5-四嗪,对参与的 1,2,4,5-四嗪的调查以及对该反应的关键机制的见解进行了详细介绍。鉴于其简单性和呼吸性,该研究建立了一种在温和条件下从容易获得的起始材料简单有效一步合成 1,2,4-三嗪的新方法。尽管替代质子溶剂(例如,MeOH 与 HFIP)提供了传统的 3,6-环加成产物,但 HFIP 增强的氢键合能力独特地促进了前所未有的正式 1,4-环加成。因此,这些研究不仅代表了通过氢键催化提高杂环氮杂二烯环加成速率或效率的例子,而且也是第一个改变环加成模式(N1/N4 与 C3/C6)的例子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号