当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Proteomics Reveals UGA-Independent Misincorporation of Selenocysteine throughout the Escherichia coli Proteome
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.jproteome.0c00352 Chunlin Hao 1 , Henry H N Lam 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.jproteome.0c00352 Chunlin Hao 1 , Henry H N Lam 1
Affiliation
|
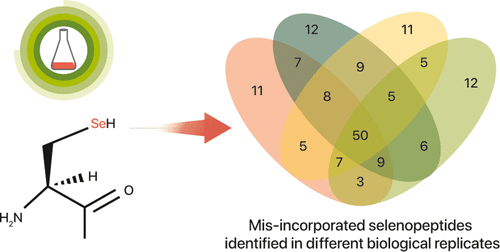
Selenocysteine is cotranslationally inserted into polypeptide chains by recoding the stop codon UGA. However, selenocysteine has also been found to be misincorporated into a small number of proteins displacing cysteines in previous studies, but such misincorporation has not yet been examined at the proteome level thoroughly. We performed label-free quantitative proteomics analysis on Escherichia coli grown in a high-selenium medium to obtain a fuller picture of selenocysteine misincorporation in its proteome. We found 139 misincorporation sites, including 54 recurred in all biological replicates, suggesting that some cysteine sites are more prone to be misincorporated than others. However, sequence and evolutionary conservation analysis showed no clear pattern among these misincorporation sites. We hypothesize that misincorporations occur randomly throughout the proteome, but the degradation rate of such misincorporated proteins varies depending on the impact of the misincorporation on protein function and stability, leading to the differential detectability of misincorporated sites by proteomics. Our hypothesis is further supported by two observations: (1) cells cultured with severely limited sulfur still retained a substantial proportion of normal cysteine counterparts of all of the found misincorporated proteins and (2) proteins involved in protein folding and proteolysis were highly upregulated in high-selenium culture.
中文翻译:

定量蛋白质组学揭示了整个大肠杆菌蛋白质组中不依赖UGA的硒代半胱氨酸的错误掺入
通过编码终止密码子UGA,将硒代半胱氨酸共翻译插入多肽链。但是,在以前的研究中,还发现硒代半胱氨酸被错误地掺入了少数取代半胱氨酸的蛋白质中,但是这种错误掺入尚未在蛋白质组学水平上得到彻底检验。我们对大肠杆菌进行了无标签定量蛋白质组学分析在高硒培养基中生长以获得蛋白质组中硒代半胱氨酸错误掺入的完整图片。我们发现了139个错误掺入位点,其中54个在所有生物学重复中都重复出现,这表明某些半胱氨酸位点比其他半胱氨酸位点更容易被错误掺入。但是,序列和进化保守性分析显示这些误掺入位点之间没有明确的模式。我们假设在整个蛋白质组中随机掺入错误掺入,但是这种掺入错误的蛋白质的降解速度取决于错误掺入对蛋白质功能和稳定性的影响,从而导致蛋白质组学对掺入错误的位点进行差异检测。我们的假设得到两个观察结果的进一步支持:
更新日期:2021-01-01
中文翻译:

定量蛋白质组学揭示了整个大肠杆菌蛋白质组中不依赖UGA的硒代半胱氨酸的错误掺入
通过编码终止密码子UGA,将硒代半胱氨酸共翻译插入多肽链。但是,在以前的研究中,还发现硒代半胱氨酸被错误地掺入了少数取代半胱氨酸的蛋白质中,但是这种错误掺入尚未在蛋白质组学水平上得到彻底检验。我们对大肠杆菌进行了无标签定量蛋白质组学分析在高硒培养基中生长以获得蛋白质组中硒代半胱氨酸错误掺入的完整图片。我们发现了139个错误掺入位点,其中54个在所有生物学重复中都重复出现,这表明某些半胱氨酸位点比其他半胱氨酸位点更容易被错误掺入。但是,序列和进化保守性分析显示这些误掺入位点之间没有明确的模式。我们假设在整个蛋白质组中随机掺入错误掺入,但是这种掺入错误的蛋白质的降解速度取决于错误掺入对蛋白质功能和稳定性的影响,从而导致蛋白质组学对掺入错误的位点进行差异检测。我们的假设得到两个观察结果的进一步支持:











































 京公网安备 11010802027423号
京公网安备 11010802027423号