当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of Al3+ Dimerization in Alkaline Solutions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02660 Maxime Pouvreau 1 , Ernesto Martinez-Baez 2 , Mateusz Dembowski 3 , Carolyn I. Pearce 4 , Gregory K. Schenter 4 , Kevin M. Rosso 4 , Aurora E. Clark 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02660 Maxime Pouvreau 1 , Ernesto Martinez-Baez 2 , Mateusz Dembowski 3 , Carolyn I. Pearce 4 , Gregory K. Schenter 4 , Kevin M. Rosso 4 , Aurora E. Clark 1
Affiliation
|
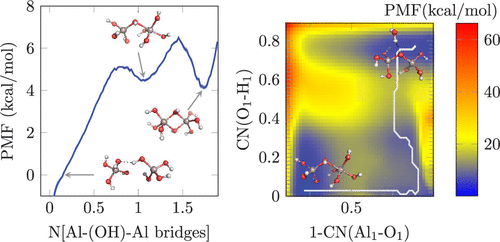
The molecular speciation of aluminum (Al3+) in alkaline solutions is fundamental to its precipitation chemistry within a number of industrial applications that include ore refinement and industrial processing of Al wastes. Under these conditions, Al3+ is predominantly Al(OH)4–, while at high [Al3+] dimeric species are also known to form. To date, the mechanism of dimer formation remains unclear and is likely influenced by complex ion···ion interactions. In the present work, we investigate a suite of potential dimerization pathways and the role of ion pairing on energetics using static DFT calculations and DFT and density functional tight binding molecular dynamics. Specific cation effects imparted by the background electrolyte cations Na+, Li+, and K+ have been examined. Our simulations predict that, when the Al species are ion-paired with either cation, the formation of the oxo-bridged Al2O(OH)62– is favored with respect to the dihydroxo-bridged Al2(OH)82–, in agreement with previous spectroscopic work. The formation of both dimers first proceeds by bridging of two monomeric units via one hydroxo ligand, leading to a labile Al2(OH)82– isomer. The effect of contact ion pairing of Li+ and K+ on the dimerization energetics is distinctly more favorable than that of Na+, which may have an effect on further oligomerization.
中文翻译:

碱性溶液中Al 3+二聚的机理
在许多工业应用中,铝(Al 3+)在碱性溶液中的分子形态对于其沉淀化学至关重要,包括矿石精炼和Al废物的工业处理。在这些条件下,铝3+是主要的Al(OH)4 - ,而在高[铝3+还已知形成二聚体。迄今为止,二聚体形成的机理尚不清楚,可能受复杂的离子···离子相互作用的影响。在当前的工作中,我们使用静态DFT计算以及DFT和密度泛函紧密结合分子动力学研究了一套潜在的二聚途径以及离子对在高能学上的作用。已经研究了由背景电解质阳离子Na +,Li +和K +赋予的特定阳离子效应。我们的模拟预测的是,当Al物质是离子配对与任一阳离子,氧代-桥连的Al的形成2 O(OH)6 2-是有利的相对于所述dihydroxo桥接的Al 2(OH)8 2–,与以前的光谱工作一致。两个二聚体的形成首先通过一个羟基配体桥接两个单体单元而进行,从而导致不稳定的Al 2(OH)8 2–异构体。Li +和K +的接触离子配对对二聚能学的影响明显优于Na +,这可能对进一步的低聚有影响。
更新日期:2020-12-21
中文翻译:

碱性溶液中Al 3+二聚的机理
在许多工业应用中,铝(Al 3+)在碱性溶液中的分子形态对于其沉淀化学至关重要,包括矿石精炼和Al废物的工业处理。在这些条件下,铝3+是主要的Al(OH)4 - ,而在高[铝3+还已知形成二聚体。迄今为止,二聚体形成的机理尚不清楚,可能受复杂的离子···离子相互作用的影响。在当前的工作中,我们使用静态DFT计算以及DFT和密度泛函紧密结合分子动力学研究了一套潜在的二聚途径以及离子对在高能学上的作用。已经研究了由背景电解质阳离子Na +,Li +和K +赋予的特定阳离子效应。我们的模拟预测的是,当Al物质是离子配对与任一阳离子,氧代-桥连的Al的形成2 O(OH)6 2-是有利的相对于所述dihydroxo桥接的Al 2(OH)8 2–,与以前的光谱工作一致。两个二聚体的形成首先通过一个羟基配体桥接两个单体单元而进行,从而导致不稳定的Al 2(OH)8 2–异构体。Li +和K +的接触离子配对对二聚能学的影响明显优于Na +,这可能对进一步的低聚有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号