当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight into Palladium-Catalyzed γ-C(sp3)–H Arylation of Alkylamines with 2-Iodobenzoic Acid: Role of the o-Carboxylate Group
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02895 Xuexiang Ma 1 , Zhe Han 2 , Chengbu Liu 1 , Dongju Zhang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02895 Xuexiang Ma 1 , Zhe Han 2 , Chengbu Liu 1 , Dongju Zhang 1
Affiliation
|
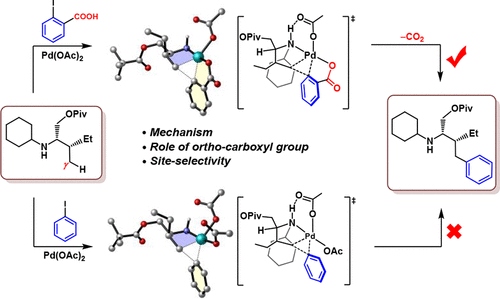
Density functional theory calculations were performed to understand the distinctly different reactivities of o-carboxylate-substituted aryl halides and pristine aryl halides toward the PdII-catalyzed γ-C(sp3)–H arylation of secondary alkylamines. It is found that, when 2-iodobenzoic acid (a representative of o-carboxylate-substituted aryl halides) is used as an aryl transfer agent, the arylation reaction is energetically favorable, while when the pristine aryl halide iodobenzene is used as the aryl transfer reagent, the reaction is kinetically difficult. Our calculations showed an operative PdII/PdIV/PdII redox cycle, which differs in the mechanistic details from the cycle proposed by the experimental authors. The improved mechanism emphasizes that (i) the intrinsic role of the o-carboxylate group is facilitating the C(sp3)−C(sp2) bond reductive elimination from PdIV rather than facilitating the oxidative addition of the aryl iodide on PdII, (ii) the decarboxylation occurs at the PdII species instead of the PdIV species, and (iii) the 1,2-arylpalladium migration proceeds via a stepwise mechanism where the reductive elimination occurs before decarboxylation, not via a concerted mechanism that merges the three processes decarboxylation, 1,2-arylpalladium migration, and C(sp3)–C(sp2) reductive elimination into one. The experimentally observed exclusive site selectivity of the reaction was also rationalized well.
中文翻译:

钯催化烷基胺与2-碘苯甲酸的γ-C(sp 3)-H芳基化反应的机理研究:邻羧酸基团的作用
进行密度泛函理论计算,以了解邻羧酸酯取代的芳基卤化物和原始芳基卤化物对仲烷基胺的Pd II催化的γ-C(sp 3)–H芳基化反应的明显不同。已经发现,当使用2-碘苯甲酸(邻-羧酸酯取代的芳基卤化物的代表)作为芳基转移剂时,芳基化反应在能量上是有利的,而当使用原始的芳基卤化物碘代苯作为芳基转移时。试剂,反应动力学困难。我们的计算结果显示术中Pd II / Pd IV / Pd II氧化还原循环,其机理细节与实验作者提出的循环不同。改进的机构强调,(i)所述的固有的作用ø -甲酸叔丁酯基团促进C(SP 3)- C(SP 2选自Pd)键还原消除IV而不是促进氧化加成在Pd芳基碘化物的II,(ii)脱羧发生在Pd II物种而不是Pd IV(iii)1,2-芳基钯的迁移是通过逐步机理进行的,其中还原消除发生在脱羧之前,而不是通过将脱羧,1,2-芳基钯迁移和C(sp 3)–C(sp 2)还原消除为一。实验观察到的反应的排他位点选择性也很合理。
更新日期:2020-12-21
中文翻译:

钯催化烷基胺与2-碘苯甲酸的γ-C(sp 3)-H芳基化反应的机理研究:邻羧酸基团的作用
进行密度泛函理论计算,以了解邻羧酸酯取代的芳基卤化物和原始芳基卤化物对仲烷基胺的Pd II催化的γ-C(sp 3)–H芳基化反应的明显不同。已经发现,当使用2-碘苯甲酸(邻-羧酸酯取代的芳基卤化物的代表)作为芳基转移剂时,芳基化反应在能量上是有利的,而当使用原始的芳基卤化物碘代苯作为芳基转移时。试剂,反应动力学困难。我们的计算结果显示术中Pd II / Pd IV / Pd II氧化还原循环,其机理细节与实验作者提出的循环不同。改进的机构强调,(i)所述的固有的作用ø -甲酸叔丁酯基团促进C(SP 3)- C(SP 2选自Pd)键还原消除IV而不是促进氧化加成在Pd芳基碘化物的II,(ii)脱羧发生在Pd II物种而不是Pd IV(iii)1,2-芳基钯的迁移是通过逐步机理进行的,其中还原消除发生在脱羧之前,而不是通过将脱羧,1,2-芳基钯迁移和C(sp 3)–C(sp 2)还原消除为一。实验观察到的反应的排他位点选择性也很合理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号