当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-Soluble α-Amino Acid Complexes of Molybdenum as Potential Antidotes for Cyanide Poisoning: Synthesis and Catalytic Studies of Threonine, Methionine, Serine, and Leucine Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-28 , DOI: 10.1021/acs.inorgchem.0c02672 Johanna M. Gretarsdottir 1 , Sigridur Jonsdottir 1 , William Lewis 2 , Trevor W. Hambley 2 , Sigridur G. Suman 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-28 , DOI: 10.1021/acs.inorgchem.0c02672 Johanna M. Gretarsdottir 1 , Sigridur Jonsdottir 1 , William Lewis 2 , Trevor W. Hambley 2 , Sigridur G. Suman 1
Affiliation
|
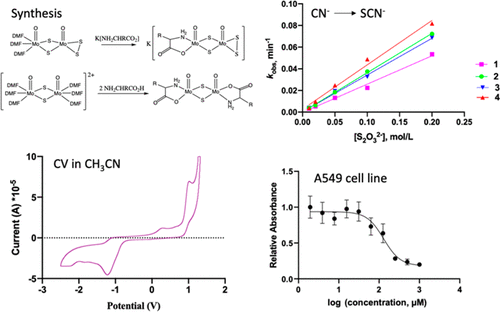
Water-soluble complexes are desirable for the aqueous detoxification of cyanide. Molybdenum complexes with α-amino acid and disulfide ligands with the formula K[(L)Mo2O2(μ-S)2(S2)] (L = leu (1), met (2), thr (3), and ser (4)) were synthesized in a reaction of [(DMF)3MoO(μ-S)2(S2)] with deprotonated α-amino acids; leu, met, thr, and ser are the carboxylate anions of l-leucine, l-methionine, l-threonine, and l-serine, respectively. Potassium salts of α-amino acids (leu (1a), met (2a), thr (3a), and ser (4a)) were prepared as precursors for complexes 1–4, respectively, by employing a nonaqueous synthesis route. The ligand exchange reaction of [Mo2O2(μ-S)2(DMF)6](I)2 with deprotonated α-amino acids afforded bis-α-amino acid complexes, [(L)2Mo2O2(μ-S)2] (6–8). A tris-α-amino acid complex, [(leu)2Mo2O2(μ-S)2(μ-leu + H)] (5; leu + H is the carboxylate anion of l-leucine with the amine protonated), formed in the reaction with leucine. 5 crystallized from methanol with a third weakly bonded leucine as a bridging bidentate carboxylate. An adduct of 8 with SCN— coordinated, 9, crystallized and was structurally characterized. Complexes 1–4 are air stable and highly water-soluble chiral molecules. Cytotoxicity studies in the A549 cell line gave IC50 values that range from 80 to 400 μM. Cyclic voltammetry traces of 1–8 show solvent-dependent irreversible electrochemical behavior. Complexes 1–4 demonstrated the ability to catalyze the reaction of thiosulfate and cyanide in vitro to exhaustively transform cyanide to thiocyanate in less than 1 h.
中文翻译:

钼的水溶性α-氨基酸配合物作为氰化物中毒的潜在解毒剂:苏氨酸,蛋氨酸,丝氨酸和亮氨酸配合物的合成和催化研究
水溶性配合物对于氰化物的水解毒是合乎需要的。分子式为K [(L)Mo 2 O 2(μ-S)2(S 2)]的具有α-氨基酸和二硫键的钼配合物(L = leu(1),met(2),thr(3) ,和ser(4))是在[(DMF)3 MoO(μ-S)2(S 2)]与去质子化的α-氨基酸反应中合成的;亮氨酸,甲硫氨酸,苏氨酸,和丝氨酸是羧酸根阴离子升l-亮氨酸,升-甲硫氨酸,升-苏氨酸,和升-丝氨酸。α氨基酸(亮氨酸(钾盐1A),满足(图2a),苏氨酸(图3a)和Ser(4A))的制备前体复合物1 - 4分别,通过采用非水合成路线。[Mo 2 O 2(μ-S)2(DMF)6 ](I)2与去质子化的α-氨基酸的配体交换反应提供了双-α-氨基酸复合物,[(L)2 Mo 2 O 2( μ-S)2 ](6 – 8)。一个三-α-氨基酸配合物,[(leu)2 Mo 2 O 2(μ-S)2(μ-leu+ H)](5; leu + H是1-亮氨酸的羧酸根阴离子,具有质子化的胺),与亮氨酸反应形成。5从甲醇中结晶出来,带有第三个弱键合的亮氨酸,作为桥接的双齿羧酸盐。的加合物8与SCN -协调,9,结晶并在结构上表征。配合物1 - 4是空气中稳定,高度水溶性的手性分子。A549细胞系的细胞毒性研究得出IC 50值范围从80到400μM。1 – 8的循环伏安曲线显示了溶剂依赖性的不可逆电化学行为。配合物1 - 4证实催化的硫代硫酸盐和氰化反应的能力在体外在小于1个小时,以彻底地变换氰化物硫氰酸盐。
更新日期:2020-12-21
中文翻译:

钼的水溶性α-氨基酸配合物作为氰化物中毒的潜在解毒剂:苏氨酸,蛋氨酸,丝氨酸和亮氨酸配合物的合成和催化研究
水溶性配合物对于氰化物的水解毒是合乎需要的。分子式为K [(L)Mo 2 O 2(μ-S)2(S 2)]的具有α-氨基酸和二硫键的钼配合物(L = leu(1),met(2),thr(3) ,和ser(4))是在[(DMF)3 MoO(μ-S)2(S 2)]与去质子化的α-氨基酸反应中合成的;亮氨酸,甲硫氨酸,苏氨酸,和丝氨酸是羧酸根阴离子升l-亮氨酸,升-甲硫氨酸,升-苏氨酸,和升-丝氨酸。α氨基酸(亮氨酸(钾盐1A),满足(图2a),苏氨酸(图3a)和Ser(4A))的制备前体复合物1 - 4分别,通过采用非水合成路线。[Mo 2 O 2(μ-S)2(DMF)6 ](I)2与去质子化的α-氨基酸的配体交换反应提供了双-α-氨基酸复合物,[(L)2 Mo 2 O 2( μ-S)2 ](6 – 8)。一个三-α-氨基酸配合物,[(leu)2 Mo 2 O 2(μ-S)2(μ-leu+ H)](5; leu + H是1-亮氨酸的羧酸根阴离子,具有质子化的胺),与亮氨酸反应形成。5从甲醇中结晶出来,带有第三个弱键合的亮氨酸,作为桥接的双齿羧酸盐。的加合物8与SCN -协调,9,结晶并在结构上表征。配合物1 - 4是空气中稳定,高度水溶性的手性分子。A549细胞系的细胞毒性研究得出IC 50值范围从80到400μM。1 – 8的循环伏安曲线显示了溶剂依赖性的不可逆电化学行为。配合物1 - 4证实催化的硫代硫酸盐和氰化反应的能力在体外在小于1个小时,以彻底地变换氰化物硫氰酸盐。











































 京公网安备 11010802027423号
京公网安备 11010802027423号