当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of an activity assay for characterizing deoxyhypusine synthase and its diverse reaction products
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-11-28 , DOI: 10.1002/2211-5463.13046 Elisabeth Kaltenegger 1 , Arunraj S Prakashrao 1 , Serhat S Çiçek 2 , Dietrich Ober 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-11-28 , DOI: 10.1002/2211-5463.13046 Elisabeth Kaltenegger 1 , Arunraj S Prakashrao 1 , Serhat S Çiçek 2 , Dietrich Ober 1
Affiliation
|
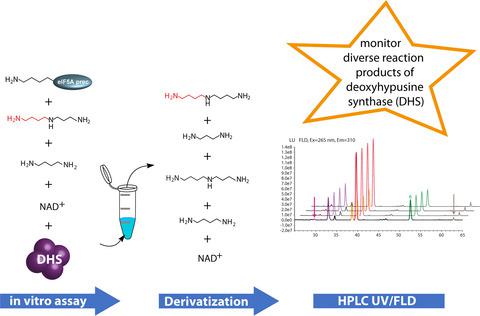
Deoxyhypusine synthase transfers an aminobutyl moiety from spermidine to the eukaryotic translation initiation factor 5A (eIF5A) in the first step of eIF5A activation. This exclusive post‐translational modification is conserved in all eukaryotes. Activated eIF5A has been shown to be essential for cell proliferation and viability. Recent reports have linked the activation of eIF5A to several human diseases. Deoxyhypusine synthase, which is encoded by a single gene copy in most eukaryotes, was duplicated in several plant lineages during evolution, the copies being repeatedly recruited to pyrrolizidine alkaloid biosynthesis. However, the function of many of these duplicates is unknown. Notably, deoxyhypusine synthase is highly promiscuous and can catalyze various reactions, often of unknown biological relevance. To facilitate in‐depth biochemical studies of this enzyme, we report here the development of a simple and robust in vitro enzyme assay. It involves precolumn derivatization of the polyamines taking part in the reaction and avoids the need for the previously used radioactively labeled tracers. The derivatized polyamines are quantified after high‐performance liquid chromatography coupled to diode array and fluorescence detectors. By performing kinetic analyses of deoxyhypusine synthase and its paralog from the pyrrolizidine alkaloid‐producing plant Senecio vernalis, we demonstrate that the assay unequivocally differentiates the paralogous enzymes. Furthermore, it detects and quantifies, in a single assay, the side reactions that occur in parallel to the main reaction. The presented assay thus provides a detailed biochemical characterization of deoxyhypusine synthase and its paralogs.
中文翻译:

开发表征脱氧马尿苷合酶及其多种反应产物的活性测定法
在 eIF5A 激活的第一步中,脱氧马尿苷合酶将氨基丁基部分从亚精胺转移至真核翻译起始因子 5A (eIF5A)。这种独特的翻译后修饰在所有真核生物中都是保守的。激活的 eIF5A 已被证明对于细胞增殖和活力至关重要。最近的报告将 eIF5A 的激活与多种人类疾病联系起来。脱氧马匹碱合酶在大多数真核生物中由单个基因拷贝编码,在进化过程中在多个植物谱系中复制,这些拷贝被反复招募到吡咯里西啶生物碱生物合成中。然而,许多这些重复的功能尚不清楚。值得注意的是,脱氧马尿苷合酶是高度混杂的,可以催化各种反应,通常具有未知的生物学相关性。为了促进对该酶的深入生化研究,我们在此报告了一种简单而强大的体外酶测定的开发。它涉及参与反应的多胺的柱前衍生化,并且避免了先前使用的放射性标记示踪剂的需要。在高效液相色谱耦合二极管阵列和荧光检测器后对衍生的多胺进行定量。通过对来自生产吡咯里西啶生物碱的植物千里光的脱氧马匹碱合酶及其旁系同源物进行动力学分析,我们证明该测定明确地区分了旁系同源酶。此外,它还可以在一次测定中检测和量化与主反应同时发生的副反应。因此,所提出的测定提供了脱氧马尿苷合酶及其旁系同源物的详细生化特征。
更新日期:2021-01-04
中文翻译:

开发表征脱氧马尿苷合酶及其多种反应产物的活性测定法
在 eIF5A 激活的第一步中,脱氧马尿苷合酶将氨基丁基部分从亚精胺转移至真核翻译起始因子 5A (eIF5A)。这种独特的翻译后修饰在所有真核生物中都是保守的。激活的 eIF5A 已被证明对于细胞增殖和活力至关重要。最近的报告将 eIF5A 的激活与多种人类疾病联系起来。脱氧马匹碱合酶在大多数真核生物中由单个基因拷贝编码,在进化过程中在多个植物谱系中复制,这些拷贝被反复招募到吡咯里西啶生物碱生物合成中。然而,许多这些重复的功能尚不清楚。值得注意的是,脱氧马尿苷合酶是高度混杂的,可以催化各种反应,通常具有未知的生物学相关性。为了促进对该酶的深入生化研究,我们在此报告了一种简单而强大的体外酶测定的开发。它涉及参与反应的多胺的柱前衍生化,并且避免了先前使用的放射性标记示踪剂的需要。在高效液相色谱耦合二极管阵列和荧光检测器后对衍生的多胺进行定量。通过对来自生产吡咯里西啶生物碱的植物千里光的脱氧马匹碱合酶及其旁系同源物进行动力学分析,我们证明该测定明确地区分了旁系同源酶。此外,它还可以在一次测定中检测和量化与主反应同时发生的副反应。因此,所提出的测定提供了脱氧马尿苷合酶及其旁系同源物的详细生化特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号