当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the Pressure Generated by Thermite Reactions Using Stress‐Altered Aluminum Particles
Propellants, Explosives, Pyrotechnics ( IF 1.8 ) Pub Date : 2020-11-30 , DOI: 10.1002/prep.202000221 Alan Williams 1 , Islam Shancita 1 , Igor Altman 2 , Nobumichi Tamura 3 , Michelle L. Pantoya 1
Propellants, Explosives, Pyrotechnics ( IF 1.8 ) Pub Date : 2020-11-30 , DOI: 10.1002/prep.202000221 Alan Williams 1 , Islam Shancita 1 , Igor Altman 2 , Nobumichi Tamura 3 , Michelle L. Pantoya 1
Affiliation
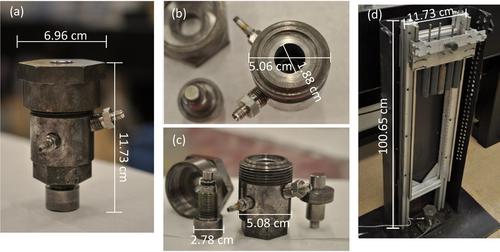
|
This study examines pressure build‐up and decay in thermites upon impact ignition and interprets reactivity based on the holistic pressure history. The thermite is a mixture of aluminum (Al) combined with bismuth trioxide (Bi2O3) powder. Four different Al particles sizes were examined that ranged from 100 nm to 18.5 μm mean diameter and for each size, two different Al powder treatments were examined: stress‐altered compared to untreated, as‐received Al powder. Stress‐altered Al powders have been shown to be more reactive, such that the stress‐altered Al powder thermites offer a metric for analyzing thermite reactivity in terms of pressure development compared to untreated Al powder. In a binary thermite system, multiple phase changes and interface chemistry influence the transient pressure response during reaction. Results reveal three key pressure metrics that need consideration specifically for thermite combustion: (1) delay time to peak pressure, (2) peak pressure, and (3) decay after peak pressure. Our experiments show that a lower peak pressure corresponds with higher thermite reactivity because aluminum consumption of oxygen generated by decomposing solid oxidizer reduces the peak pressure. Faster rates of reaction consume oxygen at higher rates such that pressure development becomes more limited than less reactive thermites and the result is a lower peak pressure. This conclusion is opposite of traditional studies using metal fuels with a gaseous environment that typically show higher peak pressures correspond with greater reactivity.
中文翻译:

改变应力的铝颗粒引起的铝热反应产生的压力
这项研究检查了冲击点火时铝热剂中的压力积累和衰减,并根据整体压力历史解释了反应性。铝热剂是铝(Al)与三氧化二铋(Bi 2 O 3)粉末。检查了四种平均粒径范围从100 nm到18.5μm的Al颗粒,对于每种尺寸,均检查了两种不同的Al粉处理:与未处理的未经处理的Al粉相比,应力改变了。应力改变的铝粉已显示出更高的反应活性,因此与未经处理的铝粉相比,应力改变的铝粉铝酸盐提供了一种在压力发展方面分析铝酸盐反应性的指标。在二元铝热体系中,多个相变和界面化学会影响反应过程中的瞬态压力响应。结果揭示了三个专门针对铝热剂燃烧需要考虑的关键压力指标:(1)达到峰值压力的延迟时间,(2)峰值压力,以及(3)达到峰值压力后的衰减。我们的实验表明,较低的峰值压力与较高的铝铁矿反应性相对应,这是因为通过分解固体氧化剂而产生的铝耗氧量降低了峰值压力。较快的反应速率会以较高的速率消耗氧气,因此压力发展比反应性较小的铝热剂更受限制,结果是峰值压力较低。这个结论与使用气体环境的金属燃料的传统研究相反,传统研究通常显示较高的峰值压力与较高的反应性相对应。
更新日期:2021-01-11
中文翻译:

改变应力的铝颗粒引起的铝热反应产生的压力
这项研究检查了冲击点火时铝热剂中的压力积累和衰减,并根据整体压力历史解释了反应性。铝热剂是铝(Al)与三氧化二铋(Bi 2 O 3)粉末。检查了四种平均粒径范围从100 nm到18.5μm的Al颗粒,对于每种尺寸,均检查了两种不同的Al粉处理:与未处理的未经处理的Al粉相比,应力改变了。应力改变的铝粉已显示出更高的反应活性,因此与未经处理的铝粉相比,应力改变的铝粉铝酸盐提供了一种在压力发展方面分析铝酸盐反应性的指标。在二元铝热体系中,多个相变和界面化学会影响反应过程中的瞬态压力响应。结果揭示了三个专门针对铝热剂燃烧需要考虑的关键压力指标:(1)达到峰值压力的延迟时间,(2)峰值压力,以及(3)达到峰值压力后的衰减。我们的实验表明,较低的峰值压力与较高的铝铁矿反应性相对应,这是因为通过分解固体氧化剂而产生的铝耗氧量降低了峰值压力。较快的反应速率会以较高的速率消耗氧气,因此压力发展比反应性较小的铝热剂更受限制,结果是峰值压力较低。这个结论与使用气体环境的金属燃料的传统研究相反,传统研究通常显示较高的峰值压力与较高的反应性相对应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号