当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Artificial Intelligence Applied to the Rapid Identification of New Antimalarial Candidates with Dual‐Stage Activity
ChemMedChem ( IF 3.6 ) Pub Date : 2020-11-27 , DOI: 10.1002/cmdc.202000685 Marilia N N Lima 1 , Joyce V B Borba 1, 2 , Gustavo C Cassiano 2, 3 , Melina Mottin 1 , Sabrina S Mendonça 1 , Arthur C Silva 1 , Kaira C P Tomaz 2 , Juliana Calit 4 , Daniel Y Bargieri 4 , Fabio T M Costa 2 , Carolina H Andrade 1, 2
ChemMedChem ( IF 3.6 ) Pub Date : 2020-11-27 , DOI: 10.1002/cmdc.202000685 Marilia N N Lima 1 , Joyce V B Borba 1, 2 , Gustavo C Cassiano 2, 3 , Melina Mottin 1 , Sabrina S Mendonça 1 , Arthur C Silva 1 , Kaira C P Tomaz 2 , Juliana Calit 4 , Daniel Y Bargieri 4 , Fabio T M Costa 2 , Carolina H Andrade 1, 2
Affiliation
|
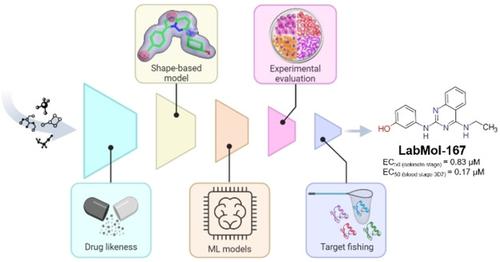
Increasing reports of multidrug‐resistant malaria parasites urge the discovery of new effective drugs with different chemical scaffolds. Protein kinases play a key role in many cellular processes such as signal transduction and cell division, making them interesting targets in many diseases. Protein kinase 7 (PK7) is an orphan kinase from the Plasmodium genus, essential for the sporogonic cycle of these parasites. Here, we applied a robust and integrative artificial intelligence‐assisted virtual‐screening (VS) approach using shape‐based and machine learning models to identify new potential PK7 inhibitors with in vitro antiplasmodial activity. Eight virtual hits were experimentally evaluated, and compound LabMol‐167 inhibited ookinete conversion of Plasmodium berghei and blood stages of Plasmodium falciparum at nanomolar concentrations with low cytotoxicity in mammalian cells. As PK7 does not have an essential role in the Plasmodium blood stage and our virtual screening strategy aimed for both PK7 and blood‐stage inhibition, we conducted an in silico target fishing approach and propose that this compound might also inhibit P. falciparum PK5, acting as a possible dual‐target inhibitor. Finally, docking studies of LabMol‐167 with P. falciparum PK7 and PK5 proteins highlighted key interactions for further hit‐to lead optimization.
中文翻译:

人工智能应用于快速识别具有双阶段活性的新型抗疟候选药物
越来越多的关于耐多药疟疾寄生虫的报道促使人们发现具有不同化学支架的新型有效药物。蛋白激酶在信号转导和细胞分裂等许多细胞过程中起着关键作用,使它们成为许多疾病的重要靶点。蛋白激酶 7 (PK7) 是一种来自疟原虫属的孤儿激酶,对这些寄生虫的孢子原循环至关重要。在这里,我们使用基于形状和机器学习模型应用了强大且综合的人工智能辅助虚拟筛选 (VS) 方法来识别具有体外抗疟原虫活性的新的潜在 PK7 抑制剂。实验评估了 8 次虚拟命中,化合物 LabMol-167 抑制了伯氏疟原虫的ookinete 转化和血液阶段的恶性疟原虫以纳摩尔浓度在哺乳动物细胞中具有低细胞毒性。由于 PK7 在疟原虫血液阶段没有重要作用,而我们的虚拟筛选策略旨在同时抑制 PK7 和血液阶段,我们进行了一种计算机模拟目标捕鱼方法,并提出该化合物也可能抑制恶性疟原虫PK5,起到作用作为一种可能的双靶点抑制剂。最后,LabMol-167 与恶性疟原虫PK7 和 PK5 蛋白的对接研究突出了进一步命中先导优化的关键相互作用。
更新日期:2020-11-27
中文翻译:

人工智能应用于快速识别具有双阶段活性的新型抗疟候选药物
越来越多的关于耐多药疟疾寄生虫的报道促使人们发现具有不同化学支架的新型有效药物。蛋白激酶在信号转导和细胞分裂等许多细胞过程中起着关键作用,使它们成为许多疾病的重要靶点。蛋白激酶 7 (PK7) 是一种来自疟原虫属的孤儿激酶,对这些寄生虫的孢子原循环至关重要。在这里,我们使用基于形状和机器学习模型应用了强大且综合的人工智能辅助虚拟筛选 (VS) 方法来识别具有体外抗疟原虫活性的新的潜在 PK7 抑制剂。实验评估了 8 次虚拟命中,化合物 LabMol-167 抑制了伯氏疟原虫的ookinete 转化和血液阶段的恶性疟原虫以纳摩尔浓度在哺乳动物细胞中具有低细胞毒性。由于 PK7 在疟原虫血液阶段没有重要作用,而我们的虚拟筛选策略旨在同时抑制 PK7 和血液阶段,我们进行了一种计算机模拟目标捕鱼方法,并提出该化合物也可能抑制恶性疟原虫PK5,起到作用作为一种可能的双靶点抑制剂。最后,LabMol-167 与恶性疟原虫PK7 和 PK5 蛋白的对接研究突出了进一步命中先导优化的关键相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号