当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Masked Alkyne Equivalents for the Synthesis of Mechanically Interlocked Polyynes**
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-30 , DOI: 10.1002/anie.202013623 Przemyslaw Gawel 1, 2 , Steffen L. Woltering 1 , Yaoyao Xiong 1 , Kirsten E. Christensen 1 , Harry L. Anderson 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-30 , DOI: 10.1002/anie.202013623 Przemyslaw Gawel 1, 2 , Steffen L. Woltering 1 , Yaoyao Xiong 1 , Kirsten E. Christensen 1 , Harry L. Anderson 1
Affiliation
|
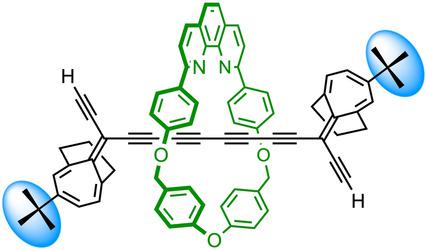
Polyyne polyrotaxanes, encapsulated cyclocarbon catenanes and other fascinating mechanically interlocked carbon‐rich architectures should become accessible if masked alkyne equivalents (MAEs) can be developed that are large enough to prevent unthreading of a macrocycle, and that can be cleanly unmasked under mild conditions. Herein, we report the synthesis of a new bulky MAE based on t‐butylbicyclo[4.3.1]decatriene. This MAE was used to synthesize a polyyne [2]rotaxane and a masked‐polyyne [3]rotaxane by Cadiot–Chodkiewicz coupling. Glaser cyclo‐oligomerization of the [2]rotaxane gave masked cyclocarbon catenanes. The unmasking behavior of the catenanes and rotaxanes was tested by photolysis at a range of UV wavelengths. Photochemical unmasking did not proceed cleanly enough to prepare extended encapsulated polyyne polyrotaxanes. We highlight the scope and challenges involved with this approach to interlocked carbon‐rich architectures.
中文翻译:

机械联锁的聚炔炔的掩盖炔烃当量**
如果可以开发出足够大的掩盖炔烃等效物(MAE)来防止大环的脱螺纹,并且可以在温和条件下干净地掩盖,则应该可以使用Polyyne聚轮烷,封装的环碳链烷烃和其他引人入胜的机械联锁的富碳结构。在此,我们报告了基于t的新的大型MAE的综合叔丁基双环[4.3.1]十碳三烯。该MAE用于通过Cadiot–Chodkiewicz偶联合成聚炔[2]轮烷和带掩盖的聚炔[3]轮烷。[2]轮烷的Glaser环低聚反应可得到掩蔽的环碳链烷烃。通过在一定范围的紫外线波长下进行光解测试,测定了链烷和轮烷的不掩盖行为。光化学脱掩膜没有进行得足够干净以制备扩展的封装的聚炔聚轮烷。我们重点介绍了这种方法用于连锁的富碳架构所涉及的范围和挑战。
更新日期:2020-11-30
中文翻译:

机械联锁的聚炔炔的掩盖炔烃当量**
如果可以开发出足够大的掩盖炔烃等效物(MAE)来防止大环的脱螺纹,并且可以在温和条件下干净地掩盖,则应该可以使用Polyyne聚轮烷,封装的环碳链烷烃和其他引人入胜的机械联锁的富碳结构。在此,我们报告了基于t的新的大型MAE的综合叔丁基双环[4.3.1]十碳三烯。该MAE用于通过Cadiot–Chodkiewicz偶联合成聚炔[2]轮烷和带掩盖的聚炔[3]轮烷。[2]轮烷的Glaser环低聚反应可得到掩蔽的环碳链烷烃。通过在一定范围的紫外线波长下进行光解测试,测定了链烷和轮烷的不掩盖行为。光化学脱掩膜没有进行得足够干净以制备扩展的封装的聚炔聚轮烷。我们重点介绍了这种方法用于连锁的富碳架构所涉及的范围和挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号