当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics and Infrared Spectrocopy of Monomeric and Dimeric Wild Type and Mutant Insulin
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.jpcb.0c08048 Seyedeh Maryam Salehi 1 , Debasish Koner 1 , Markus Meuwly 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.jpcb.0c08048 Seyedeh Maryam Salehi 1 , Debasish Koner 1 , Markus Meuwly 1
Affiliation
|
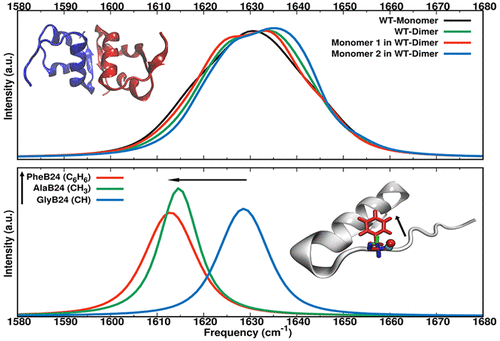
The infrared spectroscopy and dynamics of −CO labels in wild type and mutant insulin monomer and dimer are characterized from molecular dynamics simulations using validated force fields. It is found that the spectroscopy of monomeric and dimeric forms in the region of the amide-I vibration differs for residues B24–B26 and D24–D26, which are involved in dimerization of the hormone. Also, the spectroscopic signatures change for mutations at position B24 from phenylalanine, which is conserved in many organisms and is known to play a central role in insulin aggregation, to alanine or glycine. Using three different methods to determine the frequency trajectories (solving the nuclear Schrödinger equation on an effective 1-dimensional potential energy curve, using instantaneous normal modes, and using parametrized frequency maps) leads to the same overall conclusions. The spectroscopic response of monomeric WT and mutant insulin differs from that of their respective dimers, and the spectroscopy of the two monomers in the dimer is also not identical. For the WT and F24A and F24G monomers, spectroscopic shifts are found to be ∼20 cm–1 for residues (B24–B26) located at the dimerization interface. Although the crystal structure of the dimer is that of a symmetric homodimer, dynamically the two monomers are not equivalent on the nanosecond time scale. Together with earlier work on the thermodynamic stability of the WT and the same mutants, it is concluded that combining computational and experimental infrared spectroscopy provides a potentially powerful way to characterize the aggregation state and dimerization energy of modified insulins.
中文翻译:

单体和二聚体野生型和突变型胰岛素的动力学和红外光谱
使用验证的力场从分子动力学模拟中表征了野生型和突变型胰岛素单体和二聚体中-CO标记物的红外光谱和动力学。发现在酰胺-I振动区域内,单体和二聚体形式的光谱对于涉及激素二聚化的残基B24-B26和D24-D26是不同的。同样,光谱特征改变为苯丙氨酸在B24位置的突变,苯丙氨酸在许多生物中都是保守的,并且已知在胰岛素聚集中起着核心作用,变为丙氨酸或甘氨酸。使用三种不同的方法确定频率轨迹(使用瞬时法向模式在有效的一维势能曲线上求解核薛定ding方程,以及使用参数化的频率图)得出相同的总体结论。单体WT和突变型胰岛素的光谱响应与它们各自二聚体的光谱响应不同,并且二聚体中两种单体的光谱学也不相同。对于WT和F24A和F24G单体,发现光谱位移约为20 cm对于二聚化界面处的残基(B24-B26)为–1。尽管二聚体的晶体结构是对称同二聚体的晶体结构,但动态地,这两种单体在纳秒级的时间尺度上并不相等。连同有关WT和相同突变体的热力学稳定性的早期工作一起,得出的结论是,将计算和实验红外光谱法结合起来可提供一种潜在的有效方式来表征修饰胰岛素的聚集状态和二聚能。
更新日期:2020-12-31
中文翻译:

单体和二聚体野生型和突变型胰岛素的动力学和红外光谱
使用验证的力场从分子动力学模拟中表征了野生型和突变型胰岛素单体和二聚体中-CO标记物的红外光谱和动力学。发现在酰胺-I振动区域内,单体和二聚体形式的光谱对于涉及激素二聚化的残基B24-B26和D24-D26是不同的。同样,光谱特征改变为苯丙氨酸在B24位置的突变,苯丙氨酸在许多生物中都是保守的,并且已知在胰岛素聚集中起着核心作用,变为丙氨酸或甘氨酸。使用三种不同的方法确定频率轨迹(使用瞬时法向模式在有效的一维势能曲线上求解核薛定ding方程,以及使用参数化的频率图)得出相同的总体结论。单体WT和突变型胰岛素的光谱响应与它们各自二聚体的光谱响应不同,并且二聚体中两种单体的光谱学也不相同。对于WT和F24A和F24G单体,发现光谱位移约为20 cm对于二聚化界面处的残基(B24-B26)为–1。尽管二聚体的晶体结构是对称同二聚体的晶体结构,但动态地,这两种单体在纳秒级的时间尺度上并不相等。连同有关WT和相同突变体的热力学稳定性的早期工作一起,得出的结论是,将计算和实验红外光谱法结合起来可提供一种潜在的有效方式来表征修饰胰岛素的聚集状态和二聚能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号