当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vibrationally Induced Conformational Isomerization and Tunneling in Pyrrole-2-Carboxylic Acid
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.jpca.0c09141 José P. L. Roque 1 , Archna Sharma 1, 2 , Mário T. S. Rosado 1 , Rui Fausto 1 , Igor Reva 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.jpca.0c09141 José P. L. Roque 1 , Archna Sharma 1, 2 , Mário T. S. Rosado 1 , Rui Fausto 1 , Igor Reva 1
Affiliation
|
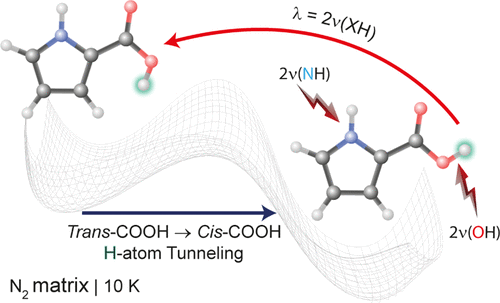
The conformational behavior of carboxylic acids has attracted considerable attention, as it can be used as a gateway for the study of more complex phenomena. Here, we present an experimental and computational study of pyrrole-2-carboxylic acid (PCA) conformational space and the vibrational characterization of the compound by infrared spectroscopy. The possibility of promoting conformational transformations using selective vibrational excitation of the 2ν(OH) and 2ν(NH) stretching overtones is explored. Two conformers, exhibiting the cis configuration of the COOH group (O═C–O–H dihedral angle near 0°) and differing by the orientation of the carboxylic group with respect to the pyrrole ring (i.e., showing either a cis or a trans NCC═O arrangement), were found to coexist initially for the compound isolated in a cryogenic nitrogen matrix, in an 86:14 ratio, and were characterized by infrared spectroscopy. A third conformer, with the COOH group in the trans configuration, was produced, in situ, by narrowband near-infrared (NIR) excitation of the most stable PCA form (with a cis NCC═O moiety). The photogenerated PCA conformer was found to decay back to the most stable PCA form, by H-atom quantum mechanical tunneling, with a characteristic half-life time of ∼10 min in the nitrogen matrix at 10 K. Tunneling rates were theoretically estimated and compared for the observed isomerization of pyrrole-2-carboxylic acid and for the structurally similar furan-2-carboxylic acid. This comparison showcases the effect of small modifications in the potential energy surface and the implications of quantum tunneling for the stability of short-living species.
中文翻译:

吡咯-2-羧酸的振动诱导构象异构化和隧穿
羧酸的构象行为已引起广泛关注,因为它可以用作研究更复杂现象的途径。在这里,我们介绍了吡咯-2-羧酸(PCA)构象空间和该化合物的红外光谱振动表征的实验和计算研究。探索了使用2ν(OH)和2ν(NH)拉伸泛音的选择性振动激发来促进构象转变的可能性。两个构象异构体,表现出COOH基团的顺式构型(O═C–OH–H二面角接近0°),并且羧基相对于吡咯环的取向不同(即,显示顺式或反式)NCC = O排列)最初与低温氮基质中分离出的化合物以86:14的比例共存,并通过红外光谱进行了表征。通过最稳定的PCA形式(带有顺式)的窄带近红外(NIR)激发原位生成具有反式构型的COOH基团的第三个构象异构体NCC = O部分)。通过H原子量子机械隧穿,发现光生PCA构象异构体会退回到最稳定的PCA形式,在10 K的氮基质中具有约10分钟的特征半衰期。理论上估计并比较了隧穿速率用于观察到的吡咯-2-羧酸的异构化和结构相似的呋喃-2-羧酸。该比较显示了势能表面的微小修改的影响以及量子隧穿对短命物种稳定性的影响。
更新日期:2020-12-10
中文翻译:

吡咯-2-羧酸的振动诱导构象异构化和隧穿
羧酸的构象行为已引起广泛关注,因为它可以用作研究更复杂现象的途径。在这里,我们介绍了吡咯-2-羧酸(PCA)构象空间和该化合物的红外光谱振动表征的实验和计算研究。探索了使用2ν(OH)和2ν(NH)拉伸泛音的选择性振动激发来促进构象转变的可能性。两个构象异构体,表现出COOH基团的顺式构型(O═C–OH–H二面角接近0°),并且羧基相对于吡咯环的取向不同(即,显示顺式或反式)NCC = O排列)最初与低温氮基质中分离出的化合物以86:14的比例共存,并通过红外光谱进行了表征。通过最稳定的PCA形式(带有顺式)的窄带近红外(NIR)激发原位生成具有反式构型的COOH基团的第三个构象异构体NCC = O部分)。通过H原子量子机械隧穿,发现光生PCA构象异构体会退回到最稳定的PCA形式,在10 K的氮基质中具有约10分钟的特征半衰期。理论上估计并比较了隧穿速率用于观察到的吡咯-2-羧酸的异构化和结构相似的呋喃-2-羧酸。该比较显示了势能表面的微小修改的影响以及量子隧穿对短命物种稳定性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号