当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Adsorption of Per- and Polyfluoroalkyl Substances (PFASs) onto Ferrihydrite Is Governed by Surface Charge
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.est.0c01646 Hugo Campos-Pereira 1 , Dan B. Kleja 1, 2 , Carin Sjöstedt 1 , Lutz Ahrens 3 , Wantana Klysubun 4 , Jon Petter Gustafsson 1, 5
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.est.0c01646 Hugo Campos-Pereira 1 , Dan B. Kleja 1, 2 , Carin Sjöstedt 1 , Lutz Ahrens 3 , Wantana Klysubun 4 , Jon Petter Gustafsson 1, 5
Affiliation
|
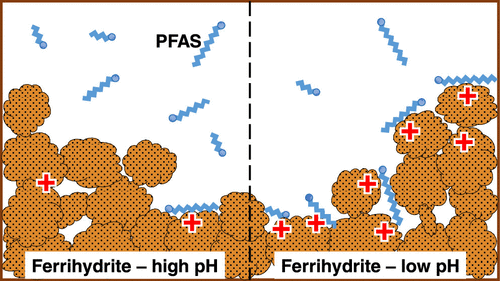
An improved quantitative and qualitative understanding of the interaction of per- and polyfluoroalkyl substances (PFASs) and short-range ordered Fe (hydr)oxides is crucial for environmental risk assessment in environments low in natural organic matter. Here, we present data on the pH-dependent sorption behavior of 12 PFASs onto ferrihydrite. The nature of the binding mechanisms was investigated by sulfur K-edge X-ray absorption near-edge structure (XANES) spectroscopy and by phosphate competition experiments. Sulfur K-edge XANES spectroscopy showed that the sulfur atom of the head group of the sulfonated PFASs retained an oxidation state of +V after adsorption. Furthermore, the XANES spectra did not indicate any involvement of inner-sphere surface complexes in the sorption process. Adsorption was inversely related to pH (p < 0.05) for all PFASs (i.e., C3–C5 and C7–C9 perfluorocarboxylates, C4, C6, and C8 perfluorosulfonates, perfluorooctane sulfonamide, and 6:2 and 8:2 fluorotelomer sulfonates). This was attributed to the pH-dependent charge of the ferrihydrite surface, as reflected in the decrease of surface ζ-potential with increasing pH. The importance of surface charge for PFAS adsorption was further corroborated by the observation that the adsorption of PFASs decreased upon phosphate adsorption in a way that was consistent with the decrease in ferrihydrite ζ-potential. The results show that ferrihydrite can be an important sorbent for PFASs with six or more perfluorinated carbons in acid environments (pH ≤ 5), particularly when phosphate and other competitors are present in relatively low concentrations.
中文翻译:

全氟和多氟烷基物质(PFASs)在水铁矿上的吸附受表面电荷控制
对全氟和多氟烷基物质(PFAS)与短程有序Fe(氢)氧化物相互作用的定量和定性认识的改进对于在天然有机物含量低的环境中进行环境风险评估至关重要。在这里,我们介绍有关12 PFAS在水铁矿上的pH依赖性吸附行为的数据。结合机理的性质是通过硫K边缘X射线吸收近边缘结构(XANES)光谱和磷酸盐竞争实验研究的。硫K边缘XANES光谱显示,磺化PFAS的头基中的硫原子在吸附后保持+ V的氧化态。此外,XANES光谱未表明内球表面复合物在吸附过程中的参与。吸附与pH值成反比(p<0.05)对于所有PFAS(即C 3 –C 5和C 7 –C 9全氟羧酸盐,C 4,C 6和C 8)全氟磺酸盐,全氟辛烷磺酰胺以及6:2和8:2氟调聚物磺酸盐)。这归因于水铁矿表面的pH依赖性电荷,反映在表面ζ电位随pH升高而降低的情况。表面电荷对于PFAS吸附的重要性得到了进一步的证实,即观察到PFAS的吸附在磷酸盐吸附后降低,其方式与三水铁矿ζ电位的降低相一致。结果表明,在酸性环境(pH≤5)中,特别是当磷酸盐和其他竞争者的浓度相对较低时,亚铁酸盐可能是具有六个或更多全氟化碳的PFAS的重要吸附剂。
更新日期:2020-12-15
中文翻译:

全氟和多氟烷基物质(PFASs)在水铁矿上的吸附受表面电荷控制
对全氟和多氟烷基物质(PFAS)与短程有序Fe(氢)氧化物相互作用的定量和定性认识的改进对于在天然有机物含量低的环境中进行环境风险评估至关重要。在这里,我们介绍有关12 PFAS在水铁矿上的pH依赖性吸附行为的数据。结合机理的性质是通过硫K边缘X射线吸收近边缘结构(XANES)光谱和磷酸盐竞争实验研究的。硫K边缘XANES光谱显示,磺化PFAS的头基中的硫原子在吸附后保持+ V的氧化态。此外,XANES光谱未表明内球表面复合物在吸附过程中的参与。吸附与pH值成反比(p<0.05)对于所有PFAS(即C 3 –C 5和C 7 –C 9全氟羧酸盐,C 4,C 6和C 8)全氟磺酸盐,全氟辛烷磺酰胺以及6:2和8:2氟调聚物磺酸盐)。这归因于水铁矿表面的pH依赖性电荷,反映在表面ζ电位随pH升高而降低的情况。表面电荷对于PFAS吸附的重要性得到了进一步的证实,即观察到PFAS的吸附在磷酸盐吸附后降低,其方式与三水铁矿ζ电位的降低相一致。结果表明,在酸性环境(pH≤5)中,特别是当磷酸盐和其他竞争者的浓度相对较低时,亚铁酸盐可能是具有六个或更多全氟化碳的PFAS的重要吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号