当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PCNA Monoubiquitination Is Regulated by Diffusion of Rad6/Rad18 Complexes along RPA Filaments
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.biochem.0c00849 Mingjie Li 1 , Bhaswati Sengupta 1 , Stephen J Benkovic 1 , Tae Hee Lee 1 , Mark Hedglin 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-27 , DOI: 10.1021/acs.biochem.0c00849 Mingjie Li 1 , Bhaswati Sengupta 1 , Stephen J Benkovic 1 , Tae Hee Lee 1 , Mark Hedglin 1
Affiliation
|
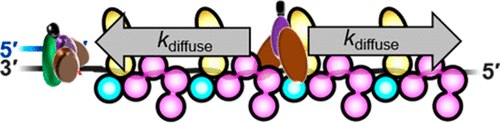
Translesion DNA synthesis (TLS) enables DNA replication through damaging modifications to template DNA and requires monoubiquitination of the proliferating cell nuclear antigen (PCNA) sliding clamp by the Rad6/Rad18 complex. This posttranslational modification is critical to cell survival following exposure to DNA-damaging agents and is tightly regulated to restrict TLS to damaged DNA. Replication protein A (RPA), the major single-strand DNA (ssDNA) binding protein complex, forms filaments on ssDNA exposed at TLS sites and plays critical yet undefined roles in regulating PCNA monoubiquitination. Here, we utilize kinetic assays and single-molecule FRET microscopy to monitor PCNA monoubiquitination and Rad6/Rad18 complex dynamics on RPA filaments, respectively. Results reveal that a Rad6/Rad18 complex is recruited to an RPA filament via Rad18·RPA interactions and randomly translocates along the filament. These translocations promote productive interactions between the Rad6/Rad18 complex and the resident PCNA, significantly enhancing monoubiquitination. These results illuminate critical roles of RPA in the specificity and efficiency of PCNA monoubiquitination and represent, to the best of our knowledge, the first example of ATP-independent translocation of a protein complex along a protein filament.
中文翻译:

PCNA 单泛素化受 Rad6/Rad18 复合物沿 RPA 细丝扩散的调节
跨损伤 DNA 合成 (TLS) 通过对模板 DNA 的破坏性修饰实现 DNA 复制,并且需要通过 Rad6/Rad18 复合物对增殖细胞核抗原 (PCNA) 滑动钳进行单泛素化。这种翻译后修饰对于暴露于 DNA 损伤剂后的细胞存活至关重要,并且受到严格调控以将 TLS 限制为受损 DNA。复制蛋白 A (RPA) 是主要的单链 DNA (ssDNA) 结合蛋白复合物,在暴露于 TLS 位点的 ssDNA 上形成细丝,并在调节 PCNA 单泛素化中发挥关键但未定义的作用。在这里,我们利用动力学分析和单分子 FRET 显微镜分别监测 RPA 细丝上的 PCNA 单泛素化和 Rad6/Rad18 复杂动力学。结果表明,Rad6/Rad18 复合物通过 Rad18·RPA 相互作用被募集到 RPA 细丝上,并沿细丝随机易位。这些易位促进了 Rad6/Rad18 复合物和常驻 PCNA 之间的有效相互作用,显着增强了单泛素化。这些结果阐明了 RPA 在 PCNA 单泛素化的特异性和效率中的关键作用,并据我们所知,代表了蛋白质复合物沿蛋白质丝的 ATP 非依赖性易位的第一个例子。
更新日期:2020-12-15
中文翻译:

PCNA 单泛素化受 Rad6/Rad18 复合物沿 RPA 细丝扩散的调节
跨损伤 DNA 合成 (TLS) 通过对模板 DNA 的破坏性修饰实现 DNA 复制,并且需要通过 Rad6/Rad18 复合物对增殖细胞核抗原 (PCNA) 滑动钳进行单泛素化。这种翻译后修饰对于暴露于 DNA 损伤剂后的细胞存活至关重要,并且受到严格调控以将 TLS 限制为受损 DNA。复制蛋白 A (RPA) 是主要的单链 DNA (ssDNA) 结合蛋白复合物,在暴露于 TLS 位点的 ssDNA 上形成细丝,并在调节 PCNA 单泛素化中发挥关键但未定义的作用。在这里,我们利用动力学分析和单分子 FRET 显微镜分别监测 RPA 细丝上的 PCNA 单泛素化和 Rad6/Rad18 复杂动力学。结果表明,Rad6/Rad18 复合物通过 Rad18·RPA 相互作用被募集到 RPA 细丝上,并沿细丝随机易位。这些易位促进了 Rad6/Rad18 复合物和常驻 PCNA 之间的有效相互作用,显着增强了单泛素化。这些结果阐明了 RPA 在 PCNA 单泛素化的特异性和效率中的关键作用,并据我们所知,代表了蛋白质复合物沿蛋白质丝的 ATP 非依赖性易位的第一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号