当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Hydrogenation Problem in Cobalt‐based Catalytic Hydroaminomethylation
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-11-27 , DOI: 10.1002/slct.202003294 Hans M. Bruijn 1, 2 , Célia Fonseca Guerra 1, 2 , Elisabeth Bouwman 1 , F. Matthias Bickelhaupt 2, 3
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-11-27 , DOI: 10.1002/slct.202003294 Hans M. Bruijn 1, 2 , Célia Fonseca Guerra 1, 2 , Elisabeth Bouwman 1 , F. Matthias Bickelhaupt 2, 3
Affiliation
|
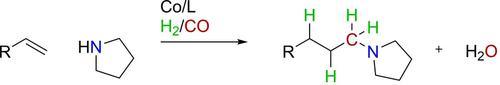
The hydroaminomethylation (HAM) reaction converts alkenes into N‐alkylated amines and has been well studied for rhodium‐ and ruthenium‐based catalytic systems. Cobalt‐based catalytic systems are able to perform the essential hydroformylation reaction, but are also known to form very active hydrogenation catalysts, therefore we examined such a system for its potential use in the HAM reaction. Thus, we have quantum‐chemically explored the hydrogenation activity of [HCo(CO)3] in model reactions with ethene, methyleneamine, formaldehyde, and vinylamine using dispersion‐corrected relativistic density functional theory at ZORA‐BLYP‐D3(BJ)/TZ2P. Our computations reveal essentially identical overall barriers for the catalytic hydrogenation of ethene, formaldehyde, and vinylamine. This strongly suggests that a cobalt‐based catalytic system will lack hydrogenation selectivity in experimental HAM reactions. Our HAM experiments with a cobalt‐based catalytic system (consisting of Co2(CO)8 as cobalt source and P(n‐Bu)3 as ligand) resulted in the formation of the desired N‐alkylated amine. However, significant amounts of hydrogenated starting material as well as alcohol (hydrogenated aldehyde) were always formed. The use of cobalt‐based catalysts in the HAM reaction to selectively form N‐alkylated amines seems therefore not feasible. This confirms our computational prediction and highlights the usefulness of state‐of‐the‐art DFT computations for guiding future experiments.
中文翻译:

钴基催化氢氨基甲基化中的氢化问题
加氢氨甲基化(HAM)反应可将烯烃转化为N烷基化胺,并且已经对基于铑和钌的催化体系进行了深入研究。钴基催化系统能够进行基本的加氢甲酰化反应,但也已知会形成非常活泼的加氢催化剂,因此,我们研究了这种系统在HAM反应中的潜在用途。因此,我们用量子化学方法研究了[HCo(CO)3]使用ZORA-BLYP-D3(BJ)/ TZ2P的色散校正相对论密度泛函理论,与乙烯,亚甲基胺,甲醛和乙烯基胺进行模型反应。我们的计算揭示了乙烯,甲醛和乙烯基胺催化加氢的基本相同的总体障碍。这有力地表明,基于钴的催化体系在实验HAM反应中将缺乏氢化选择性。我们使用基于钴的催化体系(由Co 2(CO)8作为钴源和P(n- Bu)3作为配体)进行的HAM实验导致形成所需的N烷基胺 但是,总会形成大量的氢化原料以及醇(氢化醛)。因此,在HAM反应中使用钴基催化剂选择性形成N烷基化胺似乎不可行。这证实了我们的计算预测,并强调了最新的DFT计算对于指导未来实验的有用性。
更新日期:2020-11-27
中文翻译:

钴基催化氢氨基甲基化中的氢化问题
加氢氨甲基化(HAM)反应可将烯烃转化为N烷基化胺,并且已经对基于铑和钌的催化体系进行了深入研究。钴基催化系统能够进行基本的加氢甲酰化反应,但也已知会形成非常活泼的加氢催化剂,因此,我们研究了这种系统在HAM反应中的潜在用途。因此,我们用量子化学方法研究了[HCo(CO)3]使用ZORA-BLYP-D3(BJ)/ TZ2P的色散校正相对论密度泛函理论,与乙烯,亚甲基胺,甲醛和乙烯基胺进行模型反应。我们的计算揭示了乙烯,甲醛和乙烯基胺催化加氢的基本相同的总体障碍。这有力地表明,基于钴的催化体系在实验HAM反应中将缺乏氢化选择性。我们使用基于钴的催化体系(由Co 2(CO)8作为钴源和P(n- Bu)3作为配体)进行的HAM实验导致形成所需的N烷基胺 但是,总会形成大量的氢化原料以及醇(氢化醛)。因此,在HAM反应中使用钴基催化剂选择性形成N烷基化胺似乎不可行。这证实了我们的计算预测,并强调了最新的DFT计算对于指导未来实验的有用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号