当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Ethylated Phenylcarbamoylazinane‐1,2,4‐Triazole Amides Derivatives as 15‐Lipoxygenase Inhibitors Together with Cytotoxic, ADME and Molecular Modeling Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-11-27 , DOI: 10.1002/slct.202003704 Saima Muzaffar 1 , Wardah Shahid 1 , Muhammad Saleem 1 , Muhammad Ashraf 1 , Aziz‐ur‐Rehman 2 , Bushra Bashir 1 , Mudassar Ali 1 , Mariya Al‐Rashida 3 , Bikash Baral 4 , Keshab Bhattarai 5 , Naheed Riaz 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-11-27 , DOI: 10.1002/slct.202003704 Saima Muzaffar 1 , Wardah Shahid 1 , Muhammad Saleem 1 , Muhammad Ashraf 1 , Aziz‐ur‐Rehman 2 , Bushra Bashir 1 , Mudassar Ali 1 , Mariya Al‐Rashida 3 , Bikash Baral 4 , Keshab Bhattarai 5 , Naheed Riaz 1
Affiliation
|
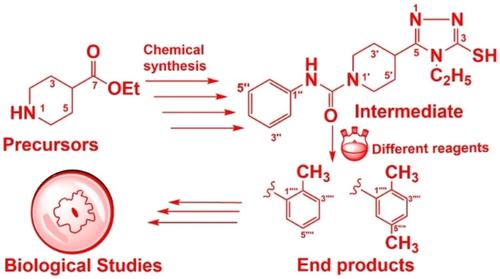
Searching the organic compound as anti‐inflammatory agent is a fruitful effort to treat inflammatory disorders such as asthma, arthritis, psoriasis, and especially cancer. These disorders can be cured by lipoxygenase (LOX) inhibitors, which have the ability to stop the development and progression of inflammation. The present research described the synthesis of fifteen new N‐alkyl/aralkyl/aryl derivatives (7 a–o) of 2‐(4‐ethyl‐5‐(1‐phenylcarbamoyl)piperidine‐4H‐1,2,4‐triazol‐3‐ylthio)acetamide by the continuous conversions of ethyl piperidine‐4‐carboxylate (a) into phenylcarbamoyl derivative (1) hydrazide (2), semicarbazide (3) and finally the N‐ethylated 5‐(1‐phenylcarbamoyl)piperidine‐1,2,4‐triazole (4). The target molecules (7 a–o) were formed by the reaction of 4 with various electrophiles (6 a–o), in methanolic potassium hydroxide. These synthetic analogues were characterized by FTIR, 1H, 13C NMR spectroscopy, EIMS, and HREIMS spectrometry. The compounds 7 a–o were screened for their inhibitory potential against 15‐lipoxygenase. Compounds 7 b, 7 e, 7 c and 7 g displayed the potent inhibitory potential (IC50 17.52±0.67, 35.61±0.81, 36.24±0.83 & 36.52±0.58 μM, respectively), whereas, moderate inhibition was shown by 7 h, 7 a, 7 d with IC50 values between 42.95±0.73 to 45.67±0.75 μM, respectively. Some compounds exhibited drug‐like characteristics due to their lower cytotoxic and good ADME profiles and supported by molecular modeling studies where one of the NH groups was found engaged through hydrogen bonding with Ala672. The carbonyl group amide and Asn554 were connected by a hydrogen bond, whereas the second NH group was also linked through hydrogen bonds with Gln363.
中文翻译:

评估作为15-脂氧合酶抑制剂的乙基化苯基氨基甲酰基叠氮烷1,2,4-三唑酰胺衍生物及其细胞毒性,ADME和分子模型研究
寻找有机化合物作为抗炎药是治疗炎症性疾病(如哮喘,关节炎,牛皮癣,尤其是癌症)的卓有成效的努力。这些疾病可通过脂氧合酶(LOX)抑制剂治愈,后者具有阻止炎症发展和进程的能力。本研究描述了15种2-(4-乙基-5-(1-苯基氨基甲酰基)哌啶-4 H -1,2,4-三唑的新N-烷基/芳烷基/芳基衍生物(7 a - o)的合成通过将哌啶-4-羧酸乙酯(a)连续转化为苯基氨基甲酰基衍生物(1)酰肼(2),氨基脲(3),最后是N-乙基化的5-(1-苯基氨基甲酰基)哌啶-1,2,4-三唑(4)。目标分子(7 a-o)是由4与各种亲电试剂(6 a - o)在甲醇氢氧化钾中反应形成的。这些合成类似物通过FTIR,1 H,13 C NMR光谱,EIMS和HREIMS光谱进行表征。筛选了化合物7 a – o对15-脂氧合酶的抑制潜力。化合物7b,7e,7c和7g显示出有效的抑制潜力(IC 50分别为17.50±0.67、35.61±0.81、36.24±0.83和36.52±0.58μM),而中度抑制表现为7 h,7 a和7 d,IC 50值为42.95± 0.73至45.67±0.75μM。一些化合物由于其较低的细胞毒性和良好的ADME特性而表现出类似于药物的特性,并受到分子建模研究的支持,其中发现一个NH基团通过与Ala672的氢键键合而结合。羰基酰胺和Asn554通过氢键连接,而第二个NH基团也通过氢键与Gln363连接。
更新日期:2020-11-27
中文翻译:

评估作为15-脂氧合酶抑制剂的乙基化苯基氨基甲酰基叠氮烷1,2,4-三唑酰胺衍生物及其细胞毒性,ADME和分子模型研究
寻找有机化合物作为抗炎药是治疗炎症性疾病(如哮喘,关节炎,牛皮癣,尤其是癌症)的卓有成效的努力。这些疾病可通过脂氧合酶(LOX)抑制剂治愈,后者具有阻止炎症发展和进程的能力。本研究描述了15种2-(4-乙基-5-(1-苯基氨基甲酰基)哌啶-4 H -1,2,4-三唑的新N-烷基/芳烷基/芳基衍生物(7 a - o)的合成通过将哌啶-4-羧酸乙酯(a)连续转化为苯基氨基甲酰基衍生物(1)酰肼(2),氨基脲(3),最后是N-乙基化的5-(1-苯基氨基甲酰基)哌啶-1,2,4-三唑(4)。目标分子(7 a-o)是由4与各种亲电试剂(6 a - o)在甲醇氢氧化钾中反应形成的。这些合成类似物通过FTIR,1 H,13 C NMR光谱,EIMS和HREIMS光谱进行表征。筛选了化合物7 a – o对15-脂氧合酶的抑制潜力。化合物7b,7e,7c和7g显示出有效的抑制潜力(IC 50分别为17.50±0.67、35.61±0.81、36.24±0.83和36.52±0.58μM),而中度抑制表现为7 h,7 a和7 d,IC 50值为42.95± 0.73至45.67±0.75μM。一些化合物由于其较低的细胞毒性和良好的ADME特性而表现出类似于药物的特性,并受到分子建模研究的支持,其中发现一个NH基团通过与Ala672的氢键键合而结合。羰基酰胺和Asn554通过氢键连接,而第二个NH基团也通过氢键与Gln363连接。











































 京公网安备 11010802027423号
京公网安备 11010802027423号