当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduced type‐A carbohydrate‐binding module interactions to cellulose I leads to improved endocellulase activity
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-11-27 , DOI: 10.1002/bit.27637 Bhargava Nemmaru , Nicholas Ramirez 1 , Cindy J Farino 2 , John M Yarbrough 3 , Nicholas Kravchenko 1 , Shishir P S Chundawat 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-11-27 , DOI: 10.1002/bit.27637 Bhargava Nemmaru , Nicholas Ramirez 1 , Cindy J Farino 2 , John M Yarbrough 3 , Nicholas Kravchenko 1 , Shishir P S Chundawat 1
Affiliation
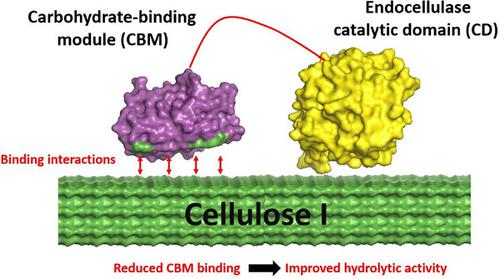
|
Dissociation of nonproductively bound cellulolytic enzymes from cellulose is hypothesized to be a key rate‐limiting factor impeding cost‐effective biomass conversion to fermentable sugars. However, the role of carbohydrate‐binding modules (CBMs) in enabling nonproductive enzyme binding is not well understood. Here, we examine the subtle interplay of CBM binding and cellulose hydrolysis activity for three models type‐A CBMs (Families 1, 3a, and 64) tethered to multifunctional endoglucanase (CelE) on two distinct cellulose allomorphs (i.e., cellulose I and III). We generated a small library of mutant CBMs with varying cellulose affinity, as determined by equilibrium binding assays, followed by monitoring cellulose hydrolysis activity of CelE–CBM fusion constructs. Finally, kinetic binding assays using quartz crystal microbalance with dissipation were employed to measure CBM adsorption and desorption rate constants  and
and  , respectively, towards nanocrystalline cellulose derived from both allomorphs. Overall, our results indicate that reduced CBM equilibrium binding affinity towards cellulose I alone, resulting from increased desorption rates (
, respectively, towards nanocrystalline cellulose derived from both allomorphs. Overall, our results indicate that reduced CBM equilibrium binding affinity towards cellulose I alone, resulting from increased desorption rates ( ) and reduced effective adsorption rates (
) and reduced effective adsorption rates ( ), is correlated to overall improved endocellulase activity. Future studies could employ similar approaches to unravel the role of CBMs in nonproductive enzyme binding and develop improved cellulolytic enzymes for industrial applications.
), is correlated to overall improved endocellulase activity. Future studies could employ similar approaches to unravel the role of CBMs in nonproductive enzyme binding and develop improved cellulolytic enzymes for industrial applications.
中文翻译:

减少 A 型碳水化合物结合模块与纤维素 I 的相互作用导致内切纤维素酶活性提高
据推测,非生产性结合的纤维素分解酶与纤维素的解离是阻碍具有成本效益的生物质转化为可发酵糖的关键限速因素。然而,碳水化合物结合模块(CBM)在促成非生产性酶结合中的作用尚不清楚。在这里,我们研究了三种模型 A 型 CBM(家族 1、3a 和 64)在两种不同的纤维素同种异形体(即纤维素 I 和 III)上与多功能内切葡聚糖酶 (CelE) 结合的 CBM 结合和纤维素水解活性的微妙相互作用. 我们生成了一个小的突变 CBM 文库,其具有不同的纤维素亲和力,通过平衡结合试验确定,然后监测 CelE-CBM 融合构建体的纤维素水解活性。最后, 和
和 ,分别针对衍生自两种同种异形体的纳米晶纤维素。总体而言,我们的结果表明,由于解吸率(
,分别针对衍生自两种同种异形体的纳米晶纤维素。总体而言,我们的结果表明,由于解吸率( )增加和有效吸附率(
)增加和有效吸附率( )降低,CBM 对单独纤维素 I 的平衡结合亲和力降低,这与整体改善的内切纤维素酶活性相关。未来的研究可以采用类似的方法来揭示 CBM 在非生产性酶结合中的作用,并开发用于工业应用的改进的纤维素分解酶。
)降低,CBM 对单独纤维素 I 的平衡结合亲和力降低,这与整体改善的内切纤维素酶活性相关。未来的研究可以采用类似的方法来揭示 CBM 在非生产性酶结合中的作用,并开发用于工业应用的改进的纤维素分解酶。
更新日期:2020-11-27
 and
and  , respectively, towards nanocrystalline cellulose derived from both allomorphs. Overall, our results indicate that reduced CBM equilibrium binding affinity towards cellulose I alone, resulting from increased desorption rates (
, respectively, towards nanocrystalline cellulose derived from both allomorphs. Overall, our results indicate that reduced CBM equilibrium binding affinity towards cellulose I alone, resulting from increased desorption rates ( ) and reduced effective adsorption rates (
) and reduced effective adsorption rates ( ), is correlated to overall improved endocellulase activity. Future studies could employ similar approaches to unravel the role of CBMs in nonproductive enzyme binding and develop improved cellulolytic enzymes for industrial applications.
), is correlated to overall improved endocellulase activity. Future studies could employ similar approaches to unravel the role of CBMs in nonproductive enzyme binding and develop improved cellulolytic enzymes for industrial applications.
中文翻译:

减少 A 型碳水化合物结合模块与纤维素 I 的相互作用导致内切纤维素酶活性提高
据推测,非生产性结合的纤维素分解酶与纤维素的解离是阻碍具有成本效益的生物质转化为可发酵糖的关键限速因素。然而,碳水化合物结合模块(CBM)在促成非生产性酶结合中的作用尚不清楚。在这里,我们研究了三种模型 A 型 CBM(家族 1、3a 和 64)在两种不同的纤维素同种异形体(即纤维素 I 和 III)上与多功能内切葡聚糖酶 (CelE) 结合的 CBM 结合和纤维素水解活性的微妙相互作用. 我们生成了一个小的突变 CBM 文库,其具有不同的纤维素亲和力,通过平衡结合试验确定,然后监测 CelE-CBM 融合构建体的纤维素水解活性。最后,
 和
和 ,分别针对衍生自两种同种异形体的纳米晶纤维素。总体而言,我们的结果表明,由于解吸率(
,分别针对衍生自两种同种异形体的纳米晶纤维素。总体而言,我们的结果表明,由于解吸率( )增加和有效吸附率(
)增加和有效吸附率( )降低,CBM 对单独纤维素 I 的平衡结合亲和力降低,这与整体改善的内切纤维素酶活性相关。未来的研究可以采用类似的方法来揭示 CBM 在非生产性酶结合中的作用,并开发用于工业应用的改进的纤维素分解酶。
)降低,CBM 对单独纤维素 I 的平衡结合亲和力降低,这与整体改善的内切纤维素酶活性相关。未来的研究可以采用类似的方法来揭示 CBM 在非生产性酶结合中的作用,并开发用于工业应用的改进的纤维素分解酶。









































 京公网安备 11010802027423号
京公网安备 11010802027423号