Water Research ( IF 11.4 ) Pub Date : 2020-11-27 , DOI: 10.1016/j.watres.2020.116680 Jing Zhao , Chii Shang , Xinran Zhang , Xin Yang , Ran Yin
|
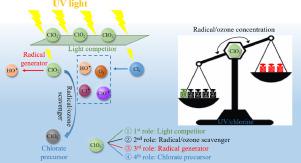
Chlorine dioxide (ClO2) has emerged as a promising alternative to free chlorine for water disinfection and/or pre-oxidation due to its reduced yields of chlorinated disinfection byproducts. ClO2 decomposes to form chlorite (ClO2−), which influences the following advanced oxidation processes (AOPs) for micropollutant abatement in drinking water. This study aims at investigating the effects of ClO2− on the concentrations of reactive species (e.g., radicals and ozone) and on the formation of chlorate in the UV/chlorine AOP. Results showed that the concentration of ClO· in the UV/chlorine process remarkably decreased by 98.20–100.00% in the presence of ClO2− at concentration of 0.1–1.0 mg·L−1 as NaClO2. The concentrations of HO· and ozone decreased by 42.71–65.42% and by 22.02–64.31%, respectively, while the concentration of Cl· was less affected (i.e., 31.00–36.21% reduction). The overall concentrations of the reactive species were differentially impacted by ClO2−’s multiple roles in the process. UV photolysis of ClO2− generated HO· but not Cl·, ClO· or ozone under the drinking water relevant conditions. ClO2− also competed with chlorine for UV photons but this effect was minor (< 1.0%). The radicals/ozone scavenging by ClO2− outcompeted the above two to lead to the overall decreasing concentrations of the reactive species, in consistency with the kinetic model predicted trends. ClO2− reacted with radicals and ozone to form chlorate (ClO3−) but not perchlorate (ClO4−). HO· played a dominant role in ClO3− formation. The findings improved the fundamental understanding on micropollutant abatement and inorganic byproduct formation by the UV/chlorine process and other AOPs in ClO2−-containing water.
中文翻译:

亚氯酸盐对游离氯的紫外线光解过程中自由基和臭氧浓度以及氯酸盐形成的多重作用
由于二氧化氯(ClO 2)降低了氯化消毒副产物的收率,因此已成为水消毒和/或预氧化中游离氯的有前途的替代品。CLO 2层分解形成氯酸钠(CLO 2 - ),这影响饮用水下列高级氧化处理(高级氧化)为微量污染物消减。这项研究的目的是调查CLO的影响2 -上的反应性物质(例如,自由基和臭氧)的浓度和在UV /氯AOP形成氯酸盐。结果表明,CLO的浓度•在UV /氯处理显着地CLO的存在减少了98.20-100.00%2 -浓度为0.1–1.0 mg•L -1的NaClO 2。HO •和臭氧的浓度分别降低了42.71–65.42%和22.02–64.31%,而Cl •的影响较小(即降低了31.00–36.21%)。所述反应性物质的总浓度被差异通过CLO影响2 -的在此过程中多个角色。在饮用水相关条件下,ClO 2 −的紫外线光解会生成HO •,但不会生成Cl •,ClO •或臭氧。CLO 2 -也与氯竞争紫外线光子,但这种影响很小(<1.0%)。通过CLO基团/臭氧清除2 - outcompeted上述两个导致所述反应性物质的总浓度降低,在一致性与动力学模型预测的趋势。CLO 2 -与自由基和臭氧形式氯酸盐反应(CLO 3 - ),但不高氯酸盐(CLO 4 - )。HO •在CLO起主导作用3 -形成。这些发现通过在CLO的UV /氯处理等的AOP改善上微量污染物减少和无机副产物形成基本理解2 -含水。











































 京公网安备 11010802027423号
京公网安备 11010802027423号