International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2020-11-27 , DOI: 10.1016/j.ijbiomac.2020.11.170 Imtiaz Khan , Ajmal Khan , Sobia Ahsan Halim , Majid Khan , Sumera Zaib , Balqees Essa Mohammad Al-Yahyaei , Ahmed Al-Harrasi , Aliya Ibrar
|
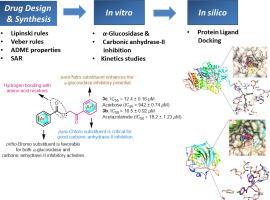
Diabetes mellitus, a progressive chronic disease, characterized by the abnormal carbohydrate metabolism is associated with severe health complications including long term dysfunction or failure of several organs, cardiovascular and micro-angiopathic problems (neuropathy, nephropathy, retinopathy). Despite the existence of diverse chemical structural libraries of α-glucosidase inhibitors, the limited diabetic treatment due to the adverse side effects such as abdominal distention, flatulence, diarrhoea, and liver damage associated with these inhibitors encourage the medicinal research community to design and develop new and potent inhibitors of α-glucosidase with better pharmacokinetic properties. In this perspective, we demonstrate the successful integration of common functional groups (ketone & ester) in one combined pharmacophore which is favorable for the formation of hydrogen bonds and other weaker interactions with the target proteins. These keto ester derivatives were screened for their α-glucosidase inhibition potential and the in vitro results revealed compound 3c as the highly active inhibitor with an IC50 value of 12.4 ± 0.16 μM compared to acarbose (IC50 = 942 ± 0.74 μM). This inhibition potency was ~76-fold higher than acarbose. Other potent compounds were 3f (IC50 = 28.0 ± 0.28 μM), 3h (IC50 = 33.9 ± 0.09 μM), 3g (IC50 = 34.1 ± 0.04 μM), and 3d (IC50 = 76.5 ± 2.0 μM). In addition, the emerging use of carbonic anhydrase inhibitors for the treatment of diabetic retinopathy (a leading cause of vision loss) prompted us to screen the keto ester derivatives for the inhibition of carbonic anhydrase-II. Compound 3b was found significantly active against carbonic anhydrase-II with an IC50 of 16.5 ± 0.92 μM (acetazolamide; IC50 = 18.2 ± 1.23 μM). Compound 3a also exhibited comparable potency with an IC50 value of 18.9 ± 1.08 μM. Several structure-activity relationship analyses depicted the influence of the substitution pattern on both the aromatic rings. Molecular docking analysis revealed the formation of several H-bonding interactions through the ester carbonyl and the nitro oxygens of 3c with the side chains of His348, Arg212 and His279 in the active pocket of α-glucosidase whereas 3b interacted with His95, -OH of Thr197, Thr198 and WAT462 in the active site of carbonic anhydrase-II. Furthermore, evaluation of ADME properties suggests the safer pharmacological profile of the tested derivatives.
中文翻译:

利用生物活性分子中的常见官能团:探究酮酯对α-葡萄糖苷酶和碳酸酐酶II酶的双重抑制潜力和计算分析
糖尿病是一种进行性慢性疾病,以碳水化合物的代谢异常为特征,伴有严重的健康并发症,包括长期功能障碍或某些器官衰竭,心血管疾病和微血管病(神经病,肾病,视网膜病)。尽管存在各种不同的化学结构库-α-葡萄糖苷酶抑制剂,但由于与这些抑制剂相关的不良副作用(如腹胀,肠胃气胀,腹泻和肝损害),糖尿病治疗受到限制,这鼓励医学研究界设计和开发新的和有效的α抑制剂-葡糖苷酶具有更好的药代动力学特性。从这个角度出发,我们证明了在一个组合药效团中常见功能基团(酮和酯)的成功整合,这有利于氢键的形成以及与目标蛋白的其他较弱的相互作用。对这些酮酯衍生物的α-葡萄糖苷酶抑制潜力进行了筛选,体外结果表明,化合物3c是一种高活性抑制剂,与阿卡波糖相比,IC 50值为12.4±0.16μM(IC 50 = 942±0.74μM)。这种抑制能力比阿卡波糖高约76倍。其他有效化合物为3f(IC 50 = 28.0±0.28μM),3h(IC 50 = 33.9±0.09μM),3g(IC 50 = 34.1±0.04μM)和3d(IC 50 = 76.5±2.0μM)。此外,碳酸酐酶抑制剂在治疗糖尿病性视网膜病变(导致视力丧失的主要原因)方面的新兴应用促使我们筛选了抑制碳酸酐酶II的酮酯衍生物。发现化合物3b具有抗碳酸酐酶II的显着活性,IC 50为16.5±0.92μM(乙酰唑胺; IC 50 = 18.2±1.23μM)。化合物3a还具有与IC 50相当的效能值为18.9±1.08μM。几种结构活性关系分析描述了取代模式对两个芳环的影响。分子对接分析显示,通过α-葡萄糖苷酶活性口袋中的His348,Arg212和His279侧链的酯羰基和3c的硝基氧形成了一些H键相互作用,而3b与Thr197的His95,-OH相互作用,碳酸酐酶-II的活性位点中的Thr198和WAT462。此外,对ADME性质的评估表明所测试衍生物的药理学特性更安全。











































 京公网安备 11010802027423号
京公网安备 11010802027423号